Diabetes: New Drug Options and Old Choices
ABSTRACT: Over the past 20 years, the treatment armamentarium for diabetes has greatly expanded: There are now many different classes of non-insulin drugs and many types of both long-and short-acting insulin now available. The newer classes of agents include disaccharidase inhibitors, thiazolidinediones, meglitinides, glucagon-like peptide one analogs, and dipeptidyl peptidase IV inhibitors. These drugs offer advantages to certain patients when used as add-on (or first-line) therapy; however, metformin remains the preferred first-line oral agent. New long-acting insulin analogs provide more constant basal insulin coverage than neutral protamine Hagedorn (NPH) insulin. Semisynthetic rapid-acting insulins help control prandial hyperglycemia with less risk of postprandial hypoglycemia than is seen with regular insulin, but the cost of analogs is much higher than for NPH or regular insulin. In addition to the many new pharmacological treatments for diabetes, the advent of continuous glucose monitoring permits relatively automated control of insulin pump administration. The evolution of diabetes treatment is continuing with active research on new agents including the sodium glucose cotransporter 2 inhibitors. New longer lasting preparations of insulin are also in sight, as is Technosphere inhaled insulin. As we welcome new treatment options, we must be well aware that advances may carry risks. The sad saga of the thiazolidinediones serves as a somber warning to be thoughtful in our use of new agents. At the same time, we should remember the significant advantages of our experience with the treatments that have proven beneficial for so many years.
Key words: diabetes, inhaled insulin, insulin analogs, biguanides, sulfonylureas, thiazolidinediones, meglitinides, glucagonlike peptide analogs, dipeptidyl peptidase IV inhibitors, continuous glucose monitoring, insulin pumps
_______________________________________________________________________________________________________________________________________________
The management of diabetes is continuing to advance. Many new agents introduced during the past 20 years have steadily improved the outlook for effective and tolerable treatment. The last decade in particular has seen tremendous progress in the pharmacotherapy of diabetes.
Although many new options for the treatment of diabetes have become available, it is vital to retain time-tested approaches that have proven track records. There are many lessons to be learned from history. In this article, I review recent developments in diabetes therapy and describe how both new and old modalities can be integrated to produce the greatest benefit for patients.
A BRIEF HISTORY OF DIABETES THERAPY
From the recognition of diabetes mellitus by the ancient Greeks until the 1900s, the only treatment for this condition was strict dietary modification. The great practitioners of diabetes care of that time, including Naunyn in Europe and Joslin in the United States, were expert at modifying caloric consumption and were even able to prolong life in type 1 diabetes, then universally fatal. The pharmacotherapy of diabetes began in the 1920s with the isolation of insulin from animal pancreas by Banting, Best, Collip and MacLeod. Insulin injections proved life-saving for children afflicted with the deadly condition, resulting in miraculous recoveries. For years, insulin remained the only pharmaceutical option for treating either type 1 or type 2 diabetes. Then, in the 1950s, the introduction of tolbutamide (a sulfonylurea) and phenformin (a biguanide) offered options for oral treatment of type 2 diabetes.1,2 Sulfonylureas stimulate endogenous insulin release by closing potassium channels in the beta cell.3 The biguanides reduce hepatic glucose production and increase peripheral glucose utilization.4
_______________________________________________________________________________________________________________________________________________
Related Content
Assessment of Psychiatric Disorders Among Older Adults With Diabetes Mellitus
Aspirin for Heart Disease Prevention in Persons With Diabetes: Is There Proof?
_______________________________________________________________________________________________________________________________________________
Setback for oral antidiabetes drugs. In 1971, the publication of the University Group Diabetes Program (UGDP) data dealt a severe blow to the development of oral antidiabetes agents.5 This large clinical study compared vascular events and mortality in treatment groups who received tolbutamide, phenformin, insulin, or placebo. The study was stopped when excess cardiovascular mortality was reported in the tolbutamide group and excess overall mortality was reported in the phenformin group. The ensuing concern led to a ban on phenformin in the United States, and the use of sulfonylureas was strongly discouraged in favor of diet and insulin therapy. The UGDP results had a chilling effect on the development of new oral hypoglycemic drugs in the United States. For a quarter century, diabetes drug development occurred primarily overseas.
Growth in use of sulfonylureas. Despite the UGDP data, patients did not want to relinquish the convenience of oral therapy in favor of daily insulin injections. The use of sulfonylureas actually increased, and by 1986, these agents had become the primary therapy for 40% of all patients with type 2 diabetes. Their use was further accelerated in the mid-1980s with the introduction of glyburide and then glipizide, both developed outside the United States.6 In the mid-1990s, an additional sulfonylurea, glimepiride, was developed and came into use.7 This agent has a slightly better safety profile than other sulfonylureas, particularly in patients with renal disease.
Metformin. This agent was synthesized in 1958 and became a primary treatment for type 2 diabetes in Europe. Because of the oral hypoglycemic controversy in the United States, it was not introduced here until the mid-1990s.8 Results of a limited series of studies in the United States led to FDA approval for metformin at a maximal dose of 2500 mg/d. This is less than the 3-g maximal daily dose in Europe.
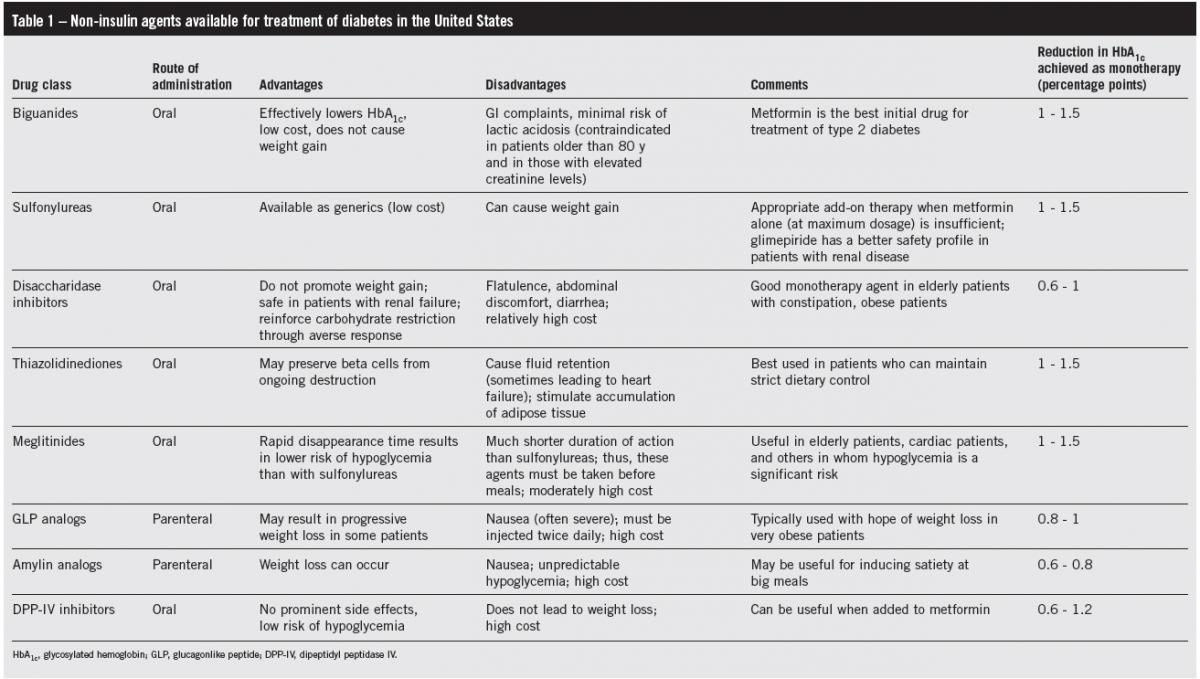
(Click to enlarge table)
Because of the risk of lactic acidosis, albeit an extremely rare event, metformin is contraindicated in patients older than 80 years and in those with elevated serum creatinine levels (Table 1). Metformin is associated with a high incidence of GI complaints: problems ranging from mild heartburn to diarrhea occur in one-third of patients. However, the symptoms usually lessen with time and in most patients are not so severe that the drug has to be stopped. An extended-release formulation of metformin has a much lower frequency of GI complaints.9 Unlike insulin and the sulfonylureas, metformin does not promote weight gain; it has therefore become the first choice for treatment of type 2 diabetes and is even used in obese type 1 diabetic patients to reduce insulin resistance.
Disaccharidase inhibitors and thiazolidinediones. Following the successful introduction of metformin in the US market, pharmaceutical manufacturers substantially expanded diabetes drug research resulting in many new treatment options:
Disaccharidase inhibitors. Two agents in this class, acarbose and miglitol, were introduced in the mid-1990s.10 Disaccharidase inhibitors effectively compensate for defective early-phase insulin release in patients with type 2 diabetes by slowing the absorption of monosaccharides in the intestine. The efficacy of disaccharidase inhibitors is limited by the adverse reactions caused by a large quantity of non-absorbed disaccharides in the intestinal tract, resulting in flatulence, abdominal discomfort, and diarrhea. However, in elderly patients, many of whom have constipation, the GI effects of the disaccharidase inhibitors may actually be beneficial.
Thiazolidinediones. The advent of the peroxisome proliferator-activated receptor (PPAR) agents constituted a major theoretical advance in diabetes treatment, generating great excitement, but this story has had a most unhappy ending. The first of these agents to be released for marketing was troglitazone, in 1997.11 Rosiglitazone and pioglitazone soon followed. All 3 agents belong to the class of PPAR agents known as thiazolidinediones (TZDs).12,13 TZDs reduce peripheral insulin resistance, primarily through an effect on adipose tissue. There is also evidence that TZDs may preserve beta cells from ongoing deterioration.14 Troglitazone was the first TZD to be approved. Within the year, the emergence of serious cases of liver toxicity led to discontinuance of the drug in Europe.15 Unfortunately, it took 3 more years, and 90 deaths due to liver failure, for troglitazone to be withdrawn from use in the United States.
Pioglitazone and rosiglitazone, agents in the same thiazolidinedione class, were not hepatotoxic and were approved for use in 1999.16 However, these drugs promote adipogenesis.17 Thin patients who adhere to dietary therapy do well with these drugs, but obese patients often gain considerable weight. The TZD class also promotes significant fluid retention, which can lead to congestive heart failure.18,19 In 2005, the first dual-PPAR agonist, muraglitazar, received a recommendation for approval by a US Food and Drug Administration (FDA) Advisory Committee. This endorsement proved to be a major embarrassment when a Cleveland Clinic group led by Steve Nissen, using the same data submitted to the FDA, showed that death, myocardial infarction (MI), or stroke occurred in twice as many muraglitazar-treated patients compared with patients in the combined placebo and pioglitazone treatment groups.20 The FDA quickly reversed their course to approval of muraglitazar. In 2007, Nissen and Wolski published a meta-analysis of trials of rosiglitazone showing that the odds ratio for MI with this agent as compared to the control population was 1.43 (P 5 .03), and the odds ratio for death from cardiovascular causes was 1.64 (P 5 .06).20
Rosiglitazone has been effectively removed from use by FDA intervention. The issue of bladder cancer has been raised as a risk of pioglitazone recently. In the original submission to the FDA, the manufacturer noted bladder cancer in rats treated with high-dose pioglitazone. This finding was dismissed as a finding related to the species. Now, a decade after marketing, several large epidemiologic studies suggest an increased incidence of human bladder cancer, related to increasing duration of exposure to the drug.21 Furthermore, the TZDs have now been implicated as promoting osteoporosis and macular edema.22,23
(Continued on next page)
NEW AGENTS
Meglitinides. Agents in this class (repaglinide and nateglinide) stimulate endogenous insulin secretion by closing potassium channels in the beta cell, a mechanism of action similar to that of the sulfonylureas.3,24 These agents must be taken before meals and have a much shorter duration of action than do the sulfonylureas. The short half-life of the meglitinides results in a lower incidence of hypoglycemia than is seen with the sulfonylureas, an advantage in elderly patients and those with coronary disease.
Glucagonlike peptide analogs. A new class of agents based on glucagonlike peptide 1 (GLP-1) has now been in use for 5 years. This intestinal hormone has multiple effects, including stimulation of insulin secretion, suppression of glucagon levels, slowing of gastric emptying, and an early satiety effect (suggesting the possibility of weight loss).25 Exenatide was approved for use as a GLP-1 agonist in 2005. The cumulative result of therapy with exenatide, a reptilian-derived analog of GLP-1, is a lowering of postprandial glucose levels. The degree to which exenatide lowers glycosylated hemoglobin (HbA1c) values is modest—about 1 percentage point, but the finding of progressive weight loss in several limited studies of exenatide has led to great enthusiasm for the use of this drug.26 However, exenatide is associated with significant adverse effects. At least 40% of patients receiving exenatide have nausea, often severe; this symptom may account for part of the observed loss of weight. Liraglutide emerged as a once a day injection with somewhat fewer gastrointestinal symptoms.27
The most serious concern to emerge related to the GLP-1 agonists is that of pancreatitis. It was only after exenatide had been marketed for 2 years that reports of fatal pancreatitis emerged, reported primarily by clinical practioners.28 Despite the arguably beneficial effects on diabetes and hyperlipidemia, the possible tendency to pancreatitis has led to increasing concern about this class of agents as well as the dipeptidyl peptidase IV (DPP-IV) class, which increase endogenous GLP-1 levels.
A further issue concerning GLP-1 agonists is related to the thyroid gland. Benign and malignant tumors of the thyroid are more common in the diabetic population. In preclinical studies in rats, liraglutide promoted C-cell hyperplasia and an increased incidence of thyroid neoplasms. Human studies initially showed an increase in calcitonin levels in liraglutide-treated patients over 1 year. Extension of the studies to 2 years suggests that this effect diminishes with time, but there is concern long-term exposure to GLP-1 agonists may promote the development of thyroid neoplasms in patients with diabetes.29
Exenatide must be administered twice daily. Liraglutide is a once daily injection. Recently, an extended-release formulation of exenatide has been approved and marketed, providing the benefits of GLP-1 for a period of 1 week after injection.30 There are several other extended duration GLP-1 agonists, including albiglutide, currently in development.
Dipeptidyl peptidase IV inhibitors. GLP-1 is rapidly degraded in the body by the enzyme dipeptidyl peptidase IV. The DPP-IV inhibitors slow the breakdown of GLP-1, resulting in higher circulating levels. Several DPP-IV agents have been synthesized and developed for use. The first approved was sitagliptin,31 followed by saxagliptin, vildagliptin in Europe, alogliptin32 in Japan, and now linagliptin.33 Linagliptin has the advantage of hepatic metabolism and excretion, making it the agent of choice in patients with renal impairment. Unlike exenatide, the DPP-IV inhibitors are not associated with significant gastrointestinal effects, but neither do they achieve weight reduction; they are weight neutral. These agents are used primarily as an adjunct to metformin therapy; resulting in less hypoglycemia than the sulfonylureas and no weight gain and fluid retention such as are seen with TZDs. However, the overall improvement in HbA1c is relatively modest, on the order of 0.6% to 1.0%, barely approaching that of the sulfonylureas.
Amylin analogs. Amylin is a 37-amino acid polypeptide secreted by the beta cell concurrently with insulin. Like GLP-1, it slows gastric emptying and inhibits postprandial glucagon secretion. In patients with insulin-dependent diabetes, amylin secretion, like insulin concentrations, is reduced. Injection of an amylin analog, pramlintide, has been shown to lower postprandial glucose levels in both type 1 and type 2 diabetes.34 Although the reduction in HbA1c concentration seen with pramlintide use is modest, the agent has been associated with weight loss both in patients with type 1 diabetes and in those with type 2 disease.However, therapy with pramlintide has been limited by a number of adverse effects, including nausea and unpredictable hypoglycemic episodes.35
Sodium glucose cotransporter 2 inhibitors. This new drug class affects the substantial glucose metabolism occurring in the kidney. These agents lower the renal threshold for glucose resulting in considerable excretion in the urine.36 Since the excretion of glucose only occurs at serum levels above normal, sodium glucose cotransporter 2 (SGLT-2) inhibitors do not cause hypoglycemia. The reduction in HbA1c achieved with the SGLT-2 inhibitors is relatively modest, but, just as with the GLP-1 agonists, the excitement generated results from the ongoing weight loss which occurs with substantial glucose excretion. The first SGLT-2 inhibitor to near approval was dapagliflozin.37 The FDA did not proceed to approval due to findings of increased incidence of breast and bladder cancer in the original study population exposed to the agent.
NEW INSULIN OPTIONS
Long-term studies such as the United Kingdom Prospective Diabetes Study (UKPDS) suggest that oral therapy with a single agent is not adequate to achieve an acceptable HbA1c level. Typically, an individual oral hypoglycemic agent improves HbA1c values by 1 to 1.5 percentage points, depending on initial glucose control. However, in the UKPDS, there was a progressive degradation of glycemic control despite the use of additional agents.38 Insulin is more effective at lowering glucose levels and can produce reductions of up to 3 percentage points in patients whose levels are inadequately controlled with combination oral therapy.39
(Click to enlarge table)
Since the introduction of insulin in the 1920s, there have been significant advances in its formulation (Table 2). Semi-synthetic analogs have improved the timing of insulin release in several ways.
Long-acting insulins. Insulin glargine provides basal insulin coverage, producing 20 to 24 hours of essentially constant insulin levels (in contrast to the peaks that occur with neutral protamine Hagedorn [NPH] or lente insulin). The use of insulin glargine as a basal insulin has been more successful than the use of NPH insulin in reducing nocturnal hypoglycemia in both type 1 and type 2 diabetes.40,41 Insulin detemir is another long-acting analog that can provide basal insulin coverage with 1 or 2 injections a day.42
There are two new long-acting insulins on the horizon: Degludec from Novo Nordisk is an albumin bound synthetic insulin-like detemir with a 42-hour duration of actions so that it can be administered once daily or at even longer intervals.43 In comparison with glargine insulin and detemir insulin, it has shown a lower incidence of hypoglycemia. Sanofi has replied to the onset of degludec by developing a threefold concentrated formulation of glargine insulin, currently in investigation.
Rapid-acting insulins. A number of semi-synthetic rapid-acting insulins have been developed by modifying a few amino acids in the insulin peptide chain to reduce the tendency of the insulin molecule to form hexamers in solution.44 The insulins modified in this way are more rapidly absorbed after subcutaneous injection than regular insulin. The first of the rapid-acting semi-synthetic insulins was insulin lispro, which was followed by insulin aspart, and most recently, insulin glulisine.45 These insulins can be administered at the time of a meal or even after a meal and still have a rapid effect on glucose levels.45 Moreover, insulin levels fall more quickly after injection than they do with regular insulin, resulting in a reduced incidence of hypoglycemia several hours after meals.46
Inhaled insulin. Insulin has been unpopular because of the need for subcutaneous administration. Although injections with a small needle are almost painless, the prospect of puncturing the skin gives rise to significant psychological resistance in the majority of patients.37
Inhaled insulin has become a potential alternative to rapid-acting injectable insulins. The availability of pulmonary administration as an option increases the willingness of patients to accept insulin therapy. In a survey of more than 700 patients with poorly controlled type 2 diabetes, 43% of those who were offered either inhalation or subcutaneous administration were amenable to insulin treatment—in contrast to only 15% of those to whom subcutaneous administration was presented as the sole option.47 As a result of disappointing revenues from the first inhaled insulin on the market, the manufacturer unexpectedly announced on October 18, 2007 that it was discontinuing sales of the product. This decision reflected a failure in promotion and marketing rather than any perceived problem with the safety or effectiveness of inhaled insulin.
Recently data from a follow-up study of patients exposed to the first marketed inhaled insulin have been analyzed showing a higher frequency of lung cancer in smokers using inhaled insulin. At the present time, the development of inhaled insulin is proceeding at Mannkind. It is hoped that there will be renewed clinical availability of inhaled insulin, subject to long-term follow-up safety studies.
(Continued on next page)
GLUCOSE MONITORING
Self-monitoring of blood glucose has empowered patients with diabetes to take control of their own treatment.48 Glucose levels are currently measured with finger sticks at strategic times—typically before and 2 hours after meals.
Subcutaneous glucose oxidase needle electrodes have become sufficiently accurate at low glucose levels to detect impending hypoglycemia. These electrodes furnish repetitive readings of subcutaneous fluid glucose levels at frequent small intervals; they interface with detectors that provide warnings of hypoglycemia or hyperglycemia. Several devices that use this technology have been released by the FDA for marketing.49,50 Continuous glucose monitoring is indicated in patients treated with insulin who have wide and abrupt fluctuations in their blood glucose levels, particularly those who experience frequent episodes of hypoglycemia or significant postprandial hyperglycemia.
The standard of treatment of type 1 diabetes is moving rapidly to use of the insulin pump. The continuous glucose monitor is essential to provide the feedback necessary for patients to manage their own insulin regimen. With further advances in the technology expected, it is likely that the algorithms to permit direct continuous monitor directed control of insulin pump infusion will come into use.
DIETARY MANAGEMENT AND WEIGHT LOSS
The degree of postprandial glucose elevation depends primarily on the carbohydrate content of the meal.51,52 Reduction of carbohydrate ingestion can successfully reduce postprandial glucose levels and HbA1c.52 Carbohydrate counting consists of flexible adjustment of the mealtime insulin dose based on an estimate of the number of grams of carbohydrate consumed. This approach has been very effective in patients whose insulin therapy is intensively managed, including those using insulin pumps.53
Regrettably, the successes in insulin management achieved with carbohydrate counting have not been accompanied by parallel achievements in the battle against obesity, the primary contributing factor in type 2 diabetes. Caloric regulation and physical activity are very effective at preventing diabetes,54,55 but counseling has been generally unsuccessful in changing lifestyles.56
There are no truly effective agents for the treatment of obesity. The agents available, which include anorexiants and orlistat, can achieve a 6% to 10% initial reduction in weight, but this reduction is typically maintained for a relatively short period.57,58 Recently, the FDA approved two new anorexiant agents, one a combination of two generic agents, the other lorcaserin. The combination drug is burdened by the known side effects of the original components. Disquieting neoplastic changes in animal studies have raised concerns about long-term use of lorcaserin.
The most successful current treatment for obesity is bariatric surgery. Rates of substantial weight loss and remission of diabetes with surgical treatment approach 60% in some series.59 The success of this procedure has led to an explosion of surgical treatment in the obese American population. However, bariatric surgery can have significant complications and should be reserved for morbidly obese patients.60
The current popularity of exenatide and other GLP-1 agonists stems from the hope that it will facilitate long-term, progressive weight loss without intolerable adverse effects. This hope is realized in some remarkable cases but only in a minority of patients.61

MAKING BEST USE OF OLD AND NEW TREATMENTS
In the face of ongoing scientific progress, it is important not to lose sight of the proven treatments of the past. There is a tendency to simply add new agents to current therapy without maximizing the benefits of the options already available. The prospect of patients receiving 3 or more oral hypoglycemic agents in an effort to suppress hyperglycemia is troublesome, particularly when little effort has been made to address obesity, which is the main cause of type 2 diabetes in so many patients. Furthermore, the ever-expanding armamentarium of new pharmaceutical agents increases the cost of treatment and the likelihood of drug-related side effects.
The basis of the treatment of type 2 diabetes remains dietary control. The preferred oral agent is still metformin, which is available in generic form everywhere in the world, costs little, and does not promote weight gain. I favor maximizing the tolerated dose of metformin before adding additional agents. The sulfonylureas are now all available as low-cost generics and are appropriate add-on therapy when metformin alone at maximum dosage is insufficient.
Meglitinides have advantages in certain patients, such as elderly persons, in whom hypoglycemia poses a significant risk. Treatment with a disaccharidase inhibitor can also be particularly beneficial in older patients. The TZDs are now disappearing from use. The DPP-IV inhibitors are appealing because of the low incidence of associated hypoglycemia, but they are quite expensive and yield relatively small improvements in HbA1c values.
Insulin is very effective at controlling hyperglycemia but has been underutilized in patients with type 2 diabetes because of the unpopularity of injections. As a result, many patients have received multiple oral hypoglycemic agents without achieving acceptable glucose levels. If long-term trials of inhaled insulin show no detrimental pulmonary effects, in particular lung cancer, inhalation may become the predominant route of administration of mealtime insulin in patients with type 1 diabetes and in those with type 2 disease. It may become possible to use very long acting insulin, perhaps as infrequently as once weekly or even monthly.
The primary unmet need in treating type 2 diabetes remains to reverse obesity, the chief exacerbating factor in this disease. Anorexiant combinations have recently become available, but have only modest effects, and carry risks consistent with their individual components. The GLP-1 preparations are used chiefly for their potential weight loss benefit. Long duration preparations make it possible to administer this treatment once weekly and, perhaps soon, at even longer intervals. Their effect is modest but, in some cases, is beneficially progressive.
As we look to the future and admit that many agents have proven to have risks counteracting their benefits, we should recognize that progress is slow. Contrary to our belief, scientific advances rarely are revolutionary. Rather, we benefit from the continued accumulation of small improvements in what exists. Even the lifesaving development of insulin shots by Banting, Best, Collip and MacLeod was built on the prior work of others to isolate insulin form animal pancreas. We benefit today from continued research and development of new treatments for diabetes, but also from improvements in existing approaches. In a period of continued regulatory and cost restrictions on medical development, it is important to recognize and defend our ability to continue to innovate.n
REFERENCES:
1. Loubatieres A. The hypoglycemic sulfonamides: history and development of the problem from 1942 to 1955. Ann N Y Acad Sci. 1957;71:4-11.
2. Bailey CJ. Biguanides and NIDDM. Diabetes Care. 1992;15:755-772.
3. Rendell M. The role of sulphonylureas in the management of type 2 diabetes mellitus. Drugs. 2004;64:1339-1358.
4. Fery F, Plat L, Balasse EO. Effects of metformin on the pathways of glucose utilization after
oral glucose in non-insulin-dependent diabetes mellitus patients. Metabolism. 1997;46:227-233.
5. Goldner MG, Knatterud GL, Prout TE. Effects of hypoglycemic agents on vascular complication in patients with adult onset diabetes. 3. Clinical implications of UGDP results. JAMA. 1971;218:
1400-1410.
6. Prendergast BD. Glyburide and glipizide, second-generation oral sulfonylurea hypoglycemic agents. Clin Pharm. 1984;3:473-485.
7. Langtry HD, Balfour JA. Glimepiride. A review of its use in the management of type 2 diabetes
mellitus. Drugs. 1998;55:563-584.
8. DeFronzo RA, Barzilai N, Simonson DC. Mechanism of metformin action in obese and noninsulin-dependent diabetic subjects. J Clin Endocrinol Metab. 1991;73:1294-1301.
9. Schwartz S, Fonseca V, Berner B, et al. Efficacy, tolerability, and safety of a novel once-daily
extended-release metformin in patients with type 2 diabetes. Diabetes Care. 2006;29:759-764.
10. Coniff RF, Shapiro JA, Seaton TB. Long-term efficacy and safety of acarbose in the treatment of obese subjects with non-insulin-dependent diabetes mellitus. Arch Intern Med. 1994;154:
2442-2448.
11. Inzucchi SE, Maggs DG, Spollett GR, et al. Efficacy and metabolic effects of metformin and troglitazone in type II diabetes mellitus. N Engl J Med. 1998;338:867-872.
12. Wagstaff AJ, Goa KL. Rosiglitazone: a review of its use in the management of type 2 diabetes mellitus. Drugs. 2002;62:1805-1837.
13. Waugh J, Keating GM, Plosker GL, et al. Pioglitazone: a review of its use in type 2 diabetes
mellitus. Drugs. 2006;66:85-109.
14. Kahn SE, Haffner SM, Heise MA, et al. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med. 2006;355:2427-2443.
15. Gale EA.Lessons from the glitazones: a story of drug development. Lancet 2001; 357(9271):
1870-1875.
16. Marcy TR, Britton ML, Blevins SM. Second generation thiazolidinediones and hepatotoxicity.
Ann Pharmacother. 2004;38:1419-1423.
17. Kersten S. Peroxisome proliferator activated receptors and obesity. Eur J Pharmacol. 2002;440(2-3):223-234.
18. Hartung DM, Touchette DR, Bultemeier NC, Haxby DG. Risk of hospitalization for heart failure associated with thiazolidinedione therapy: a Medicaid claims-based case-control study. Pharmacotherapy. 2005;25:1329-1336.
19. Dormandy JA, Charbonnel B, Eckland DJ, et al. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial. Lancet. 2005;366:1279-1289.
20. Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007;356(24):2457-2471.
21. Azoulay L, Yin H, Filion KB, et al. The use of pioglitazone and the risk of bladder cancer in people with type 2 diabetes: nested case-control study. BMJ. 2012;344:e3645. doi: 10.1136/bmj.e3645.
22. Motola D, Piccinni C, Biagi C, et al. Cardiovascular, ocular and bone adverse reactions associated with thiazolidinediones: a disproportionality analysis of the US FDA adverse event reporting system database. Drug Saf. 2012;35(4):315-323.
23. Idris I, Warren G, Donnelly R. Association between thiazolidinedione treatment and risk of macular edema among patients with type 2 diabetes. Arch Intern Med. 2012;172(13):1005-1011.
24. Blickle JF. Meglitinide analogues: a review of clinical data focused on recent trials. Diabetes Metab. 2006;32:113-120.
25. Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and
dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet. 2006;368:1696-1705.
26. Blonde L, Klein EJ, Han J, et al. Interim analysis of the effects of exenatide treatment on A1c, weight and cardiovascular risk factors over 82 weeks in 314 overweight patients with type 2 diabetes. Diabetes Obes Metab. 2006;8:436-447.
27. Gough SC. Liraglutide: from clinical trials to clinical practice. Diabetes Obes Metab. 2012;
14(suppl 2):33-40.
28. Ayoub WA, Kumar AA, Naguib HS, Taylor HC. Exenatide-induced acute pancreatitis. Endocr Pract. 2010;16(1):80-83.
29. Elashoff M, Matveyenko AV, Gier B, et al. Pancreatitis, pancreatic, and thyroid cancer with glucagon-like peptide-1-based therapies. Gastroenterology. 2011;141(1):150-156.
30. Scott LJ. Exenatide extended-release: a review of its use in type 2 diabetes mellitus. Drugs. 2012;72(12):1679-1707.
31. Lyseng-Williamson KA. Sitagliptin. Drugs. 2007;67(4):587-597.
32. Andukuri R, Drincic A, Rendell M. Alogliptin: a new addition to the class of DPP-4 inhibitors. Diabetes Metab Syndr Obes. 2009;2:117-126.
33. Deeks ED. Linagliptin: a review of its use in the management of type 2 diabetes mellitus. Drugs. 2012;72(13):1793-1824. doi: 10.2165/11209570-000000000-00000. PMID: 22913735
34. Singh-Franco D, Robles G, Gazze D. Pramlintide acetate injection for the treatment of type 1
and type 2 diabetes mellitus. Clin Ther. 2007;29:535-562.
35. Mathews AW. An FDA reviewer battles the drug his boss approved: private letter gets Dr Misbin pulled from diabetes case but he pursues it anyway. Wall Street Journal. October 26, 2005:A1.
36. Kim Y, Babu AR. Clinical potential of sodium-glucose cotransporter 2 inhibitors in the management of type 2 diabetes. Diabetes Metab Syndr Obes. 2012;5:313-327. Epub 2012 Aug.
37. Anderson SL, Marrs JC. Dapagliflozin for the treatment of type 2 diabetes. Ann Pharmacother. 2012;46(4):590-598. Epub 2012 Mar 20.
38. Turner RC, Cull CA, Frighi V, Holman RR. Glycemic control with diet, sulfonylurea, metformin, or insulin in patients with type 2 diabetes mellitus: progressive requirement for multiple therapies (UKPDS 49): UK Prospective Diabetes Study (UKPDS) Group. JAMA. 1999;281:2005-2012.
39. Lindstrom T, Eriksson P, Olsson AG, Arnqvist HJ. Long-term improvement of glycemic control by insulin treatment in NIDDM patients with secondary failure. Diabetes Care. 1994;17:719-721.
40. Alemzadeh R, Berhe T, Wyatt DT. Flexible insulin therapy with glargine insulin improved glycemic control and reduced severe hypoglycemia among preschool-aged children with type 1 diabetes mellitus. Pediatrics. 2005;115:1320-1324.
41. Yki-Järvinen H, Dressler A, Ziemen M; HOE 901/300s Study Group. Less nocturnal hypoglycemia and better post-dinner glucose control with bedtime insulin glargine compared with bedtime NPH insulin during insulin combination therapy in type 2 diabetes. HOE 901/3002 Study Group. Diabetes Care. 2000;23:1130-1136.
42. Chapman TM, Perry CM. Insulin detemir: a review of its use in the management of type 1 and 2 diabetes mellitus. Drugs. 2004;64:2577-2595.
43. Wang F, Surh J, Kaur M. Insulin degludec as an ultralong-acting basal insulin once a day:
a systematic review. Diabetes Metab Syndr Obes. 2012;5:191-204. Epub 2012 Jul 5.
44. Hirsch IB. Insulin analogues. N Engl J Med. 2005;352:174-183.
45. Garnock-Jones KP, Plosker GL. Insulin glulisine: a review of its use in the management of diabetes mellitus. Drugs. 2009;69(8):1035-1057. doi: 10.2165/00003495-200969080-00006.
46. Holleman F, Schmitt H, Rottiers R, et al. Reduced frequency of severe hypoglycemia and coma
in well controlled IDDM patients treated with insulin lispro. The Benelux UK Insulin Lispro Study Group. Diabetes Care. 1997;20:1827-1832.
47. Freemantle N, Blonde L, Duhot D, et al. Availability of inhaled insulin promotes greater perceived acceptance of insulin therapy in patients with type 2 diabetes. Diabetes Care. 2005;28:427-428.
48. Blonde L, Karter AJ. Current evidence regarding the value of self-monitored blood glucose testing. Am J Med. 2005;118(suppl 9A):20S-26S.
49. Garg SK, Schwartz S, Edelman SV. Improved glucose excursions using an implantable real-time continuous glucose sensor in adults with type 1 diabetes. Diabetes Care. 2004;27:734-738.
50. Wong LJ, Buckingham BA, Kunselman B, et al. Extended use of a new continuous glucose monitoring system with wireless data transmission in children with type 1 diabetes mellitus. Diabetes Technol Ther. 2006;8:139-145.
51. Nuttall FQ, Gannon MC. Plasma glucose and insulin response to macronutrients in nondiabetic and NIDDM subjects. Diabetes Care. 1991;14:824-838.
52. Gutierrez M, Akhavan M, Jovanovic L, Peterson CM. Utility of a short-term 25% carbohydrate diet on improving glycemic control in type 2 diabetes mellitus. J Am Coll Nutr. 1998;17:595-600.
53. Rabasa-Lhoret R, Garon J, Langelier H, et al. Effects of meal carbohydrate content on insulin requirements in type 1 diabetic patients treated intensively with the basal-bolus (ultralente-regular) insulin regimen. Diabetes Care. 1999;22:667-673.
54. Pi-Sunyer FX. How effective are lifestyle changes in the prevention of type 2 diabetes mellitus? Nutr Rev. 2007;65:101-110.
55. Hu G, Lakka TA, Kilpelainen TO, Tuomeilehto J. Epidemiological studies of exercise in diabetes prevention. Appl Physiol Nutr Metab. 2007;32:583-595.
56. Dansinger Ml, Tatsioni A. Wong JB, et al. Meta-analysis: the effect of dietary counseling for weight loss. Ann Intern Med. 2007;147:41-50.
57. Ioannides-Demos LL, Proietto J, Tonkin AM, McNeil JJ. Safety of drug therapies used for weight loss and treatment of obesity. Drug Saf. 2006;29:277-302.
58. Padwal RS, Majumdar SR. Drug treatments for obesity: orlistat, sibutramine, and rimonabant. Lancet. 2007;369:71-77.
59. Shah M, Simha V, Garg A. Review: long-term impact of bariatric surgery on body weight, comorbidities, and nutritional status. Clin Endocrinol Metab. 2006;91:4223-4231.
60. Livingston EH. Complications of bariatric surgery. Surg Clin North Am. 2005;85:853-868.
61. Amori RE, Lau J, Pittas AG. Efficacy and safety of incretin therapy in type 2 diabetes: systematic review and meta-analysis. JAMA. 2007;298:194-206.