Abdominal Pain From Pneumatosis Intestinalis
Introduction
Pneumatosis intestinalis (PI), the presence of gas in the wall of the gastrointestinal tract, is an underrecognized cause of abdominal pain. The gas may be either subserosal or submucosal and is usually found in the colon and small bowel, but can also be present in the esophagus or stomach. PI can be a benign condition or a surgical emergency, and the course of treatment must be determined rapidly. Patients with PI commonly present with gastrointestinal symptoms, including nausea, vomiting, and abdominal pain, but some present with no symptomatology. It is a relatively uncommon condition, with an incidence in the general population estimated to be 0.03%.1,2 It more commonly affects the elderly, but can occur at any age. Currently, there are over 60 possible causative diseases that have been proposed as contributing to the condition.3 Since the first report of PI in 1754 by Duvernoy, a French pathologist, appropriate treatment options have continued to evolve.1,4 Traditionally, after a radiographic diagnosis, PI was considered a surgical emergency. More recent studies, however, have proven surgical treatment to be unnecessary in many cases, although life-threatening causes must first be ruled out.1,3,5-7
The purpose of this article is to highlight the importance of correctly identifying the underlying etiology of the PI so that the appropriate management is undertaken. We examine the case of an elderly woman who presented to the emergency department (ED) with the common reports of abdominal pain and constipation.
Case Presentation
A 68-year-old woman presented to the ED with a 1-week history of intermittent, sharp, generalized abdominal pain and constipation. The pain was described as diffuse, was not associated with food intake, and was not exacerbated or relieved by anything. The patient had not had a bowel movement for 1 week before having a normal, nonbloody bowel movement just prior to her ED presentation. She also reported a 1-day history of nausea with several episodes of nonbloody emesis. She reported no fevers, chills, dysuria, flank pain, or any prior episodes of similar abdominal pain.
The patient’s medical history was significant for coronary artery disease, hypercholesterolemia, and hypothyroidism. Her surgical history included an appendectomy, hysterectomy, and angioplasty with stent placement. Her medications included levothyroxine 50 mcg daily and simvastatin 20 mg daily.
On physical examination, the patient’s vital signs were stable, and she was found to have dry mucous membranes and decreased bowel sounds on auscultation. Her abdomen was soft and nondistended, with generalized tenderness, but no rebound or guarding on palpation; her stool was hemoccult-negative. Laboratory test results on admission showed a normal white blood cell count of 5900/µL and lactic acid level of 7.2 mg/dL, as well as normal electrolyte levels, liver function tests, and pancreatic enzyme levels. A plain x-ray of the patient’s abdomen showed gas-distended loops of small bowel. A computed tomography (CT) scan of the patient’s abdomen and pelvis showed marked distention of the large bowel from the cecum to the descending colon, including portions of the distal small bowel, with no point of obstruction (Figure 1).The CT scan also showed fatty infiltration along the ascending and proximal transverse colon with PI in the large bowel and distal small bowel.

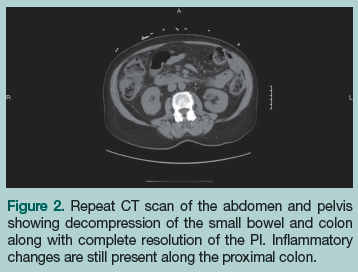
With the initial working diagnosis of PI due to ischemic bowel, the patient was instructed to receive nothing by mouth. Intravenous (IV) fluids were started, a nasogastric tube was placed, broad-spectrum IV antibiotics (piperacillin/tazobactam 3.375 g IV every 6 hours) were started empirically in the ED and continued on admission, and cardiology and surgery were consulted for surgical clearance. On day 1 of admission, the patient remained hemodynamically stable, had a bowel movement, and her abdominal pain decreased. At this point, PI due to ischemic bowel was ruled out and surgery was deemed to be no longer needed. She was started on sips of water and ice chips, which were well tolerated. On day 2 of admission, the patient had another bowel movement and her abdominal pain completely resolved. A repeat CT scan of the abdomen and pelvis showed interval bowel decompression and complete resolution of the PI, which confirmed that there was no need for surgical intervention (Figure 2). The patient was discharged to home the following day on ciprofloxacin 500 mg twice daily and metronidazole 500 mg 3 times daily for 10 days.
Discussion
PI can be classified into one of two groups: primary or secondary PI. Primary PI, also known as pneumatosis cystoides intestinalis, consists of multiple cystic collections of gas in the submucosa or subserosa of the gastrointestinal tract. Its etiology is unknown, with theories ranging from idiopathic to genetic causes.8 A possible association between autoantibodies against proliferating cell nuclear antigen with gastrointestinal dysfunction, including PI, has been demonstrated.9,10 Primary PI is usually benign and asymptomatic. Secondary PI, the more common form, is characterized by linear and/or circumferential patterns of gas. This form of PI is a sign of one of many underlying conditions, which range from benign to life-threatening. Since 1958, it has been attributed to at least 60 causative factors3 (Table). Determining which of these factors is responsible for the PI in each individual case is of the utmost importance in developing the most appropriate treatment plan. Although the exact cause of PI is often not clear, multiple theories have been proposed to explain the pathophysiology of secondary PI.1,3,7,11
 One causative theory is that PI is secondary to pulmonary diseases such as asthma, chronic obstructive pulmonary disease, chest trauma, and cystic fibrosis. In this theory, it is thought that alveolar rupture results in gas dissecting interstitially along bronchopulmonary bundles to the mediastinum and then retroperitoneally along the vascular supply of the viscera. A second theory, the mucosal disruption theory, attempts to explain the presence of gas by a mechanical breakdown, which includes PI secondary to obstruction, peptic ulcer disease, endoscopy, inflammatory bowel disease, Hirschsprung’s disease, blunt trauma, surgery, and amyloidosis. In this theory, it is thought that a mechanical break in the mucosa, along with increased intraluminal pressure, allows gas to be forced into the bowel wall. A final theory is that of increased mucosal permeability, which includes patients with AIDS, patients with collagen vascular disease, patients undergoing chemotherapy, and patients on chronic steroid therapy or different types of immunotherapy. In this theory, it is thought that the illness or treatment breaks down the mucosal permeability, allowing the gas to penetrate the bowel wall.1,3,7,11
One causative theory is that PI is secondary to pulmonary diseases such as asthma, chronic obstructive pulmonary disease, chest trauma, and cystic fibrosis. In this theory, it is thought that alveolar rupture results in gas dissecting interstitially along bronchopulmonary bundles to the mediastinum and then retroperitoneally along the vascular supply of the viscera. A second theory, the mucosal disruption theory, attempts to explain the presence of gas by a mechanical breakdown, which includes PI secondary to obstruction, peptic ulcer disease, endoscopy, inflammatory bowel disease, Hirschsprung’s disease, blunt trauma, surgery, and amyloidosis. In this theory, it is thought that a mechanical break in the mucosa, along with increased intraluminal pressure, allows gas to be forced into the bowel wall. A final theory is that of increased mucosal permeability, which includes patients with AIDS, patients with collagen vascular disease, patients undergoing chemotherapy, and patients on chronic steroid therapy or different types of immunotherapy. In this theory, it is thought that the illness or treatment breaks down the mucosal permeability, allowing the gas to penetrate the bowel wall.1,3,7,11
There have also been reports of PI in patients with pharmacological constipation, cytomegalovirus infection, candidiasis, celiac disease, burns, Clostridium difficile infection, and Cryptosporidium infection, and in persons who use certain medications such as chloral hydrate and other alkyl halides.12-20 In many of these patients, the pathophysiology is not clear, since there are several factors that need to be examined that could be the source of the PI. One known cause of PI is bowel necrosis, which includes ischemia or infarct, caustic ingestion, sepsis, and necrotizing enterocolitis. The presence of gas in the portal system and/or the bowel wall is a common sequela of ischemia.
PI can be seen on plain abdominal x-rays and ultrasonography, but it is best diagnosed by a CT scan of the abdomen. In one study, only 23% of patients with PI diagnosed on CT were shown to have radiographic evidence of PI on plain film.21 As stated previously, primary PI is characterized by cystic collections in the bowel wall, while secondary PI is characterized by linear and/or circumferential patterns of gas.
Although CT scanning is more discriminating than plain films, CT scans alone have not been shown to be predictive of which patients have true intestinal ischemia or necrosis versus primary PI or PI from benign secondary causes.21 It was traditionally thought that the evidence of portomesenteric venous gas was an ominous sign of ischemia, but this has been disproven.22 In a study published in 1990, of 11 patients with demonstrable portal venous gas, 4 had definite ischemic bowel and underwent surgical resection, 2 had questionable ischemia and underwent surgical resection, and 5 survived without resection.5 PI is most common in the colon and then, in decreasing order, the small bowel, colon and small bowel, stomach, and esophagus.21
Basic laboratory tests for a patient presenting with abdominal pain, which can be a sign of ischemic or necrotic bowel, include complete blood count with differential, basic metabolic panel, liver function tests, prothrombin time, and assessments of amylase, lipase, and lactic acid levels. Elevated lactic acid and a decreased arterial pH have been shown to correlate with diminished survival.5,21 The increased lactic acid levels correlate with an ischemic and/or necrotic bowel. An elevated white blood cell count and the presence of sepsis have also been found to be useful in determining the need for surgery.6 The utility of other basic laboratory assessments is debatable and has not demonstrated predictive value in the outcome of patients with PI.
Since most laboratory values alone are not very helpful in determining the severity of PI, there are disease severity indicators that can be used. The Acute Physiology and Chronic Health Evaluation II (APACHE II) score has been shown to be predictive of mortality in patients with PI in the intensive care unit.21 It consists of assessing 12 routine physiologic measurements, along with age and previous health status, to determine a score of 0 to 71—the higher the score, the worse the outcome.
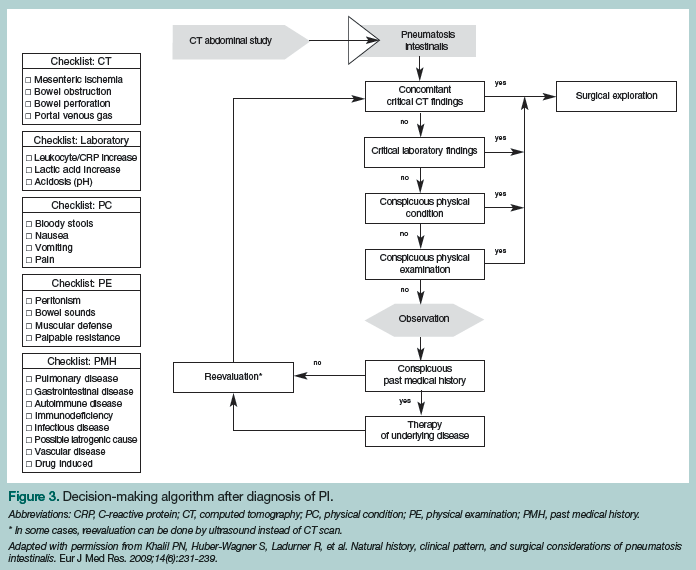
Treatment for patients with PI is as varied as its causes and is determined by the cause itself. Most cases of PI can be treated without operative intervention. After the underlying factor, whether it be constipation, Crohn’s disease, or any of the other 60 possible causes, is determined and treated, PI usually resolves. The patients who are of most concern are those who have ischemic and/or necrotic bowel, as these individuals require emergent operative intervention. Any delay in admission to the operating room could cause a significant increase in mortality risk. In some cases, it might not be a clear-cut decision, but one that needs to be made by a competent surgeon. In 2009, a treatment algorithm was developed that includes evaluating CT scan results, physical examination findings, and medical history (Figure 3).7 This may be used to support the decision-making process, rather than as an instruction guide.
In the case discussed in this article, the surgery department was immediately consulted and PI due to ischemic bowel was ruled out after examining the patient’s medical history, laboratory results, and radiographic findings while she remained hemodynamically stable with resolution of her abdominal pain. The patient had a 1-week history of constipation, her lactic acid level was in the normal range, and the CT scan showed no portal venous gas. It was therefore determined that she was not an appropriate surgical candidate; however, as with many cases of PI, the definitive etiology was not clear. Was the patient suffering from constipation, partial bowel obstruction, infection, or a combination of several pathologic conditions? Perhaps infection led to increased mucosal permeability, while the constipation led to increased intraluminal pressure with mucosal disruption. Once she was started on conservative management (antibiotics, IV fluids, and bowel rest) and had a bowel movement, the pneumatosis resolved.
Conclusion
In many cases, PI is the result of a combination of factors and not just one cause or condition. One singular and life-threatening cause of PI is ischemic or necrotic bowel, which has been proven to release gas into the bowel wall and the portal venous system. In addition, it may be that some persons are predisposed to PI, whether genetically or from an autoimmune-mediated process. There are still a lot of unanswered questions that need to be researched and answered. Whatever the cause, when a patient presents with PI, multiple factors need to be considered, including history of the present illness, the patient’s complete medical history, current medications, laboratory findings, abdominal and pelvic CT scan results, and—most importantly—findings from a thorough physical examination. Surgical emergencies and ischemic and/or necrotic bowel must be ruled out, but the majority of the time, PI is a benign finding that can be managed safely with a nonoperative approach.
The authors report no relevant financial relationships.
Dr. Bowers is a Resident, and Dr. Simmons is Assistant Professor, Department of Family and Community Medicine, Drexel University College of Medicine, Philadelphia, PA.
References
1. Ho LM, Paulson EK, Thompson WM. Pneumatosis intestinalis in the adult: benign to life-threatening causes. AJR Am J Roentgenol. 2007;188(6):1604-1613.
2. Heng Y, Schuffler MD, Haggitt RC, Rohrmann CA. Pneumatosis intestinalis: a review. Am J Gastroenterol. 1995;90(10):1747-1758.
3. Pear BL. Pneumatosis intestinalis: a review. Radiology. 1998;207(1):13-19.
4. Koss LG. Abdominal gas cysts (pneumatosis cystoides intestinorum hominis); an analysis with a report of a case and a critical review of the literature. AMA Arch Pathol. 1952;53(6):523-549.
5. Knechtle SJ, Davidoff AM, Rice RP. Pneumatosis intestinalis. Surgical management and clinical outcome. Ann Surg.1990;212(2):160-165.
6. Greenstein AJ, Nguyen SQ, Berlin A, et al. Pneumatosis intestinalis in adults: management, surgical indications, and risk factors for mortality. J Gastrointest Surg. 2007;11(10):1268-1274.
7. Khalil PN, Huber-Wagner S, Ladurner R, et al. Natural history, clinical pattern, and surgical considerations of pneumatosis intestinalis. Eur J Med Res. 2009;14(6):231-239.
8. Underwood JW, Finnis D, Scott W. Pneumatosis coli: a familial association. Br J Surg. 1978;65(1):64-65.
9. Onouchi H, Muro Y, Horigome H. Pneumatosis cystoides intestinalis associated with autoantibodies against proliferating cell nuclear antigen: comment on the article by Nojima et al. Arthritis Rheum. 1997;40(12):2279-2280.
10. Nojima Y, Mimura T, Hamasaki K, et al. Chronic intestinal pseudoobstruction associated with autoantibodies against proliferating cell nuclear antigen. Arthritis Rheum. 1996;39(5):877-879.
11. St. Peter SD, Abbas MA, Kelly KA. The spectrum of pneumatosis intestinalis. Arch Surg. 2003;138(1):68-75.
12. Prieto C, Fernandez-Urien I, Sangro B, et al. Pneumatosis coli due to pharmacological constipation. Gastrointest Endosc. 2007;65(4):710-711.
13. Chetty R, Roskell DE. Cytomegalovirus infection in the gastrointestinal tract. J Clin Pathol. 1994;47(11):968-972.
14. Andrutis KA, Riggle PJ, Kumamoto CA, Tzipori S. Intestinal lesions associated with disseminated candidiasis in an experimental animal model. J Clin Microbiol. 2000;38(6):2317-2323.
15. Altaf MA, Grunow JE. Atypical presentations of celiac disease: recurrent intussusception and pneumatosis intestinalis. Clin Pediatr (Phila). 2008;47(3):289-292.
16. Balledux J, McCurry T, Zieger M, Coleman JJ, Sood R. Pneumatosis intestinalis in a burn patient: case report and literature review. J Burn Care Res. 2006;27(3):399-403.
17. Gillett PM, Russell RK, Wilson DC, Thomas AE. C. difficile induced pneumatosis intestinalis in a neutropenic child. Arch Dis Child. 2002;87(1):85.
18. Hunter PR, Nichols G. Epidemiology and clinical features of Cryptosporidium infection in immunocompromised patients. Clin Microbiol Rev. 2002;15(1):145-154.
19. Marigold JH. Pneumatosis cystoides coli and chloral hydrate. Gut. 1998;42(6):899-900.
20. Florin TH. Alkyl halides, super hydrogen production and the pathogenesis of pneumatosis cystoides coli. Gut. 1997;41(6):778-784.
21. Morris MS, Gee AC, Cho D, et al. Management and outcome of pneumatosis intestinalis. Am J Surg. 2008;195(5):679-683.
22. Wiesner W, Mortelé KJ, Glickman JN, Ji H, Ros P. Pneumatosis intestinalis and portomesenteric venous gas in intestinal ischemia: correlation of CT findings with severity of ischemia and clinical outcome. AJR Am J Roentgenol. 2001;177(6):1319-1323.


