Recognition and Management of Autonomic Dysfunction in Patients With a Lewy Body Disorder: Part I
Lewy bodies—named for Friedrich Lewy, the German neuropathologist who discovered them circa 1912—are neuronal inclusions primarily containing alpha-synuclein proteins that develop in the brainstem, cerebral, and autonomic nervous system.1 The formation of Lewy bodies is characteristic of certain dementias, including Parkinson’s disease (PD) with dementia (PDD) and dementia with Lewy bodies (DLB). PDD and DLB demonstrate clinical and neuropathological similarities, especially in later clinical stages,2 and some researchers have proposed that Lewy body disorders (LBDs) represent a spectrum of neurodegenerative disease.2-4 For diagnostic purposes, however, the DLB Consortium states that “DLB should be diagnosed when dementia occurs before or concurrently with parkinsonism and PDD should be used to describe dementia that occurs in the context of well-established PD.”4 Although DLB and PDD are less prevalent than Alzheimer’s disease dementia and mixed/vascular dementia, they account for 10% to 20% of dementia diagnoses.2
Autonomic dysfunction, or dysautonomia, is a recognized complication of LBDs (ie, PD and DLB), and it is especially common among patients with PDD and DLB.5 Approximately 62% of patients with DLB experience significant autonomic failure,6 and dysautonomia is observed in up to 80% of patients with PD.7 Most studies of autonomic dysfunction in patients with PD have excluded individuals without dementia, making it difficult to quantify the prevalence of these events in patients with PDD. However, autopsy series in patients with PD but not dementia who had demonstrated autonomic failure have typically discovered Lewy bodies,8 and Lewy bodies are thought to be the primary cause of dysautonomia in patients with PD with and without dementia.7 Components of autonomic failure observed in patients with PD/DLB include sleep disturbances; sialorrhea (excessive secretion of saliva); excessive sweating; sexual dysfunction; temperature dysregulation; and failure of the gastrointestinal, urinary, and cardiovascular systems.7 In a study investigating the prevalence of autonomic failure in individuals with DLB, 28 of 29 patients whose disease was confirmed at autopsy had demonstrated evidence of autonomic dysfunction (ie, urinary incontinence, constipation, and orthostatic hypotension [OH]) while alive.9 It was previously thought that autonomic failure in PD and DLB occurred only in patients with late-stage disease, but more recent studies contradict this. For example, Magerkurth et al10 reported that 48% of patients with PD demonstrated symptoms of cardiovascular autonomic failure within 5 years of diagnosis.
We have divided this article into two parts; part I reviews OH in patients with PD and DLB, whereas part II—which will be published in the next issue of Clinical Geriatrics—will address other major components of cardiovascular autonomic failure. Together, the articles will review the basic pathophysiology behind cardiovascular dysautonomia, will discuss how to evaluate and manage cardiovascular dysautonomia, and will describe common disease processes associated with this cardiovascular phenomenon.
Pathophysiology of Cardiovascular Dysautonomia
The anatomic distribution of Lewy bodies appears to dictate the clinical manifestations of PD and DLB and determines whether patients will experience dysfunction of the autonomic nervous system (ANS).4,11 The ANS is a branch of the peripheral nervous system that facilitates neuronal impulses between the central nervous system and the body’s organs, helping to regulate involuntary (unconscious) functions such as contraction of smooth muscles and the cardiac muscle.7
Neurocardiovascular instability, a term that encompasses several manifestations of cardiovascular dysautonomia, including OH, vasovagal syndrome, and carotid sinus hypersensitivity, is prevalent in all common dementias.5 In patients with PDD and DLB, the evolving picture suggests that cardiovascular-related autonomic dysfunction occurs when Lewy bodies and alpha-synuclein proteins aggregate within the neurons found within the central nervous system and the peripheral nervous system. How this alpha-synuclein aggregation disrupts the individual neuron is debated. A picture is evolving, however, that suggests the autonomic manifestations of disease result from a combination of central and peripheral nervous system injuries. This includes damage to various regulating nuclei in the brain and the loss of peripheral nerves.12,13 Imaging studies, immunohistochemical staining, and functional medication studies all support the idea that cardiovascular dysautonomia in LBDs is caused, at least in part, by the loss of sympathetic and parasympathetic nerves.14-20 Researchers continue to debate whether the cumulative burden of Lewy bodies within the neural tissue of the cardiovascular system or the extent of denervation—believed to be caused by an excessive number of Lewy bodies within neural tissue—correlates with a patient’s risk of cardiovascular autonomic failure and its severity.11,17,19-23
Orthostatic Hypotension
Orthostatic hypotension is defined as a drop in systolic blood pressure (BP) of >20 mm Hg and/or a drop in diastolic BP of >10 mm Hg within 3 minutes of tilting maneuvers (passive) or maneuvering from a reclining position to a seated position to a standing position (active).12,24 The rate of OH in the general population is estimated to range from 5% to 30%.25,26 OH risk increases with age and is relatively common among older adults, many of whom are asymptomatic.5,25
OH is significantly more prevalent in patients with PD, PDD, and DLB than in normal elders and is widely recognized as a nonmotor complication of LBDs.5,25,27-29 A study by Senard and colleagues29 found that 58% of patients with PD had OH, yet only 20% of these individuals were symptomatic. Andersson et al25 reported a prevalence of OH of 69%, whereas Allan and associates5 reported a 52% rate of OH in patients with DLB and a 49% rate of OH in patients with PDD. OH may be more severe in patients with DLB compared with patients with PD,25 but studies conflict regarding the extent of correlation between the severity of OH, the patient’s age, and LBD disease duration.28-30
Untreated OH is associated with stroke, ischemic cardiac disease, cognitive decline, and increased mortality.31-36 In a study of patients >65 years of age who presented to the emergency department after briefly losing consciousness, likely as a result of a syncopal condition, OH was established as the etiology for 12.4%.37 The authors found that patients with OH were more likely than those without OH to have an existing diagnosis of PD.
Matinolli and colleagues38 showed that patients with PD and OH had increased postural sway upon standing (vs while walking) as compared with PD patients without OH; postural sway is a known risk factor for falling. Although the study concluded that increased postural sway, and not OH, was the cause of the falls, the findings suggest that patients with OH remain at an increased risk of falls.38 Another study considered the presence of OH symptoms and the duration for which the BP level remains below baseline to be greater predictors of fall risk than the magnitude of the decline in BP level.39
Diagnosing Orthostatic Hypotension
Different methods are available to evaluate patients for OH (Table 1). Research protocols commonly use passive tilt table testing or head-up tilting at an angle between 60° and 70°, but this may not be practical in the clinical setting.40-43 Although there are no standards for in-clinic testing for OH, patients can be assessed with a standard sphygmomanometer while actively moving from a reclining position to standing. For patients with an LBD, some evidence supports monitoring BP levels continuously for up to 10 minutes after tilting/standing to watch for delayed OH.25,41 An ambulatory 24-hour BP and heart rate monitor may be a convenient way to evaluate patients for OH, supine hypertension, or postprandial hypotension.12,24,44
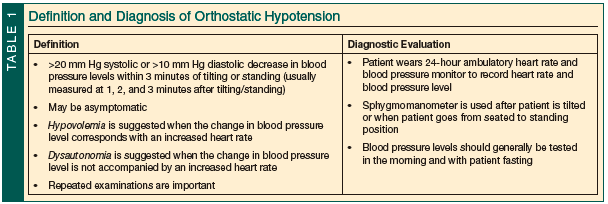
It is believed that OH occurs most often in the morning before breakfast and that this may be the time when the condition is most likely to be induced in the clinic.24 Variables discussed in various reports include testing for OH in the morning, after the patient has fasted through the night and prior to his or her taking any morning medications. Some studies recommend having the patient assume a variable supine position for 5 to 15 minutes, in a room with a steady temperature from 20°C to 22°C, prior to a position change.24,40-42,45
During an OH evaluation, a change in BP level combined with a corresponding increase in heart rate indicates hypovolemia or medication-induced OH. A change in BP level but no increase in heart rate favors a diagnosis of autonomic failure.12,24 One negative test result, however, is not sufficient to rule out OH, and practitioners should repeatedly assess patients with PD, PDD, or DLB for OH.
Managing Orthostatic Hypotension
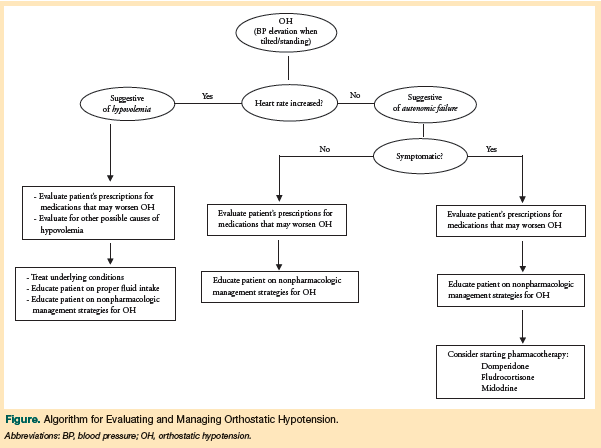
The goal of managing OH should be to reduce the severity and frequency of symptoms, thus allowing the patient to ambulate more often. Although a reduction in OH symptoms may decrease the risk of falls while walking, note that an increase in time spent ambulatory may convey a greater risk of falls due to other causes. Controlled studies to guide the clinical management of OH in patients with PD, PDD, or DLB are lacking. Expert clinical opinion and theory represent the bulk of available information, and the Figure offers a proposed algorithm for evaluating and managing OH.
Many medications prescribed to patients with PD, PDD, and DLB can induce or exacerbate OH, including antiparkinsonian medications, diuretics, alpha-blockers, oral nitrates, tricyclic antidepressants, beta-blockers, alpha-1 adrenergic receptor antagonists, calcium channel blockers, monoamine oxidase inhibitors, and catechol-O-methyltransferase inhibitors; ethyl alcohol abuse can also contribute to the onset of OH or worsen symptoms of OH.12,27,44,46-48
The first step in managing OH in patients with LBDs should be to review prescription drug use in these patients for any OH-inducing and OH-exacerbating agents. Recommended changes include decreasing dosages, eliminating medications where possible, or changing medication classes.12,24,44,49 To cause the least amount of motor change, it has been suggested that selegiline be the first of the most commonly prescribed antiparkinsonian medications to be decreased or halted.50 Although levodopa has been found to affect BP levels, this occurs primarily when patients are first starting the drug or when the dose is increased.12,51 Therefore, a diagnosis of OH in a levodopa-naïve patient with PD52,53 does not contraindicate the initiation of levodopa therapy, if the physician feels that it is clinically indicated.54
 Approaches for treating OH consist of nonpharmacologic (Table 2) and pharmacologic treatment options.12 One of the most commonly discussed nonpharmacologic therapies for OH is compression stockings. They have been shown to be effective in managing OH, but patients demonstrate a very low compliance rate, particularly those living in warmer climates.12,24,44,45 Elevating the head of the bed is an effective way to reduce nocturia (waking at night to urinate). Physical maneuvers such as crossing, elevating, or flexing one’s legs or exercise help decrease vascular pooling and increase venous return; avoiding elevated temperatures can reduce vascular capacitance.12,24,46 A small study of nonpharmacologic therapies for reducing OH in patients with PD found that the degree of improvement was subjective.45
Approaches for treating OH consist of nonpharmacologic (Table 2) and pharmacologic treatment options.12 One of the most commonly discussed nonpharmacologic therapies for OH is compression stockings. They have been shown to be effective in managing OH, but patients demonstrate a very low compliance rate, particularly those living in warmer climates.12,24,44,45 Elevating the head of the bed is an effective way to reduce nocturia (waking at night to urinate). Physical maneuvers such as crossing, elevating, or flexing one’s legs or exercise help decrease vascular pooling and increase venous return; avoiding elevated temperatures can reduce vascular capacitance.12,24,46 A small study of nonpharmacologic therapies for reducing OH in patients with PD found that the degree of improvement was subjective.45
Numerous medications have been proposed for the treatment of OH,12 with midodrine, fludrocortisone, and domperidone being the drugs most commonly discussed. However, concerns regarding polypharmacy and drug-drug interactions have led to recommendations that pharmacotherapy be reserved for patients with symptomatic OH.
Midodrine is the only medication that has evidence to support its use as a treatment for OH.55 Midodrine has a short half-life and is the preferred agent for patients with OH and supine hypertension; use of fludrocortisone, which has a long half-life, is discouraged in these patients. Because of its short half-life, midodrine can be taken in the morning, at lunch, or within 4 hours of bedtime—but no later than 5 pm—to decrease OH symptoms and will not counteract the effectiveness of antihypertensive medications taken at night.12,24,51,56 Midodrine is associated with drug-induced hypertension and/or bradycardia,12 however, and it should not be used to treat patients who are at risk for or have a history of cardiac disease.
One study found improvement in Composite Autonomic Symptom Scale and Clinical Global Impression of Change scores in patients with PD whose OH was treated with domperidone or fludrocortisone.45 In this small, randomized controlled study, patients demonstrated better tolerance of domperidone. A review of treatment options for nonmotor symptoms of PD argued for using domperidone as a first-line agent in the treatment of OH because it was associated with additional beneficial effects, including a reduction in nausea, vomiting, and gastroparesis, all of which are autonomic symptoms that patients with an LBD may experience.57 In contrast, a small French study reported that although domperidone is widely used, evidence supporting its efficacy in ameliorating OH is lacking.58 Domperidone can cause QTc prolongation, which is known to occur with LBDs.57 At present, evidence supporting the use of fludrocortisone or domperidone to manage OH in patients with PD, PDD, or DLB is insufficient.54,58 Pyridostigmine is another drug that has been investigated in this patient population and recently was reported to reduce symptoms of OH without worsening supine hypertension.59,60
When evaluating the effectiveness of a treatment regimen, it is important to consider how the medications are being taken versus how they are prescribed. For example, if the clinician instructs the patient to take midodrine with breakfast, lunch, and dinner, the patient might awaken at 9 am, eat a small breakfast at 10 am, have lunch at 12:30 pm, eat dinner at 5 pm, and go to bed at 7 pm. This meal and medication use pattern would cause plasma trough levels of the medication to peak mid-day, with resultant supine hypertension, and would counteract any nighttime antihypertensive medications. To further understand the pharmacologic causes and the therapeutic options for OH in patients with an LBD, physicians should avail themselves of the many in-depth reviews that are available.12,24,44,46,47,56
Conclusion
Autonomic dysfunction is a frequent complication of the spectrum of LBDs. OH, an aspect of this associated dysautonomia, has a high prevalence among patients with an LBD. OH is often asymptomatic, and physicians who treat patients with an LBD should be vigilant about evaluating their patients for OH. Some studies have found that OH can have a profound effect on patients, affecting long-term physical health and quality of life.
Diagnosing OH in patients with LBDs may require more than monitoring/testing BP levels in the clinic; long-term monitoring and listening to the patient’s subjective symptom complaints may also be necessary. Adequate treatment of OH in patients with PD, PDD, or DLB is often elusive,12 and may require multiple agents. Polypharmacy has its own pitfalls, however, with one study of Veterans Affairs patients demonstrating a positive correlation between the number of medications used and the risk of OH47; several in-depth reviews regarding pharmacologic causes of and therapies for OH exist.12,24,44,46,47,56
Selecting the appropriate therapeutic options combines the physician’s best understanding of the patient’s global clinical picture and quality of life, the physician’s level of comfort with the medication(s), and the patient’s ability to tolerate the medications. It is important to remember that a treatment intended to improve the patient’s quality of life may have a profound effect on the patient’s global well-being that goes beyond improving numbers. In the second part of this article, we will discuss other signs and symptoms associated with LBDs.
Disclaimer:
The views expressed in this article are those of the author(s) and do not necessarily reflect the official policy or position of the Department of the Navy, Department of Defense, or the United States Government.
Copyright Statement:
The authors are military service members. This work was prepared as part of their official duties. Title 17 USC 105 provides that “Copyright protection under this title is not available for any work of the United States Government.” Title 17 USC 101 defines a United States Government work as a work prepared by a military service member or employee of the United States Government as part of that person’s official duties.
The authors report no relevant financial relationships.
Dr. Walsh recently completed his family medicine internship with Naval Hospital Jacksonville, FL, and is currently a flight surgery student with the Naval Aerospace Medicine Institute in Pensacola, FL. Dr. Unwin is Vice Chair, Department of Family Medicine, Uniformed Services University, Bethesda, MD.
References
1. McKeith IG, Burn D, O’Brien J, Perry R, Perry E. Dementia with Lewy bodies. In: Davis KL, Charney D, Coyle JT, Nemeroff C, eds. Neuropsychopharmacology: The Fifth Generation of Progress. Philadelphia, PA: Lippincott Williams & Wilkins; 2002:1301-1315.
2. Gold G. Dementia with Lewy bodies: clinical diagnosis and therapeutic approach. Front Neurol Neurosci. 2009;24:107-113.
3. Andersson M, Zetterberg H, Minthon L, Blennow K, Londos E. The cognitive profile and CSF biomarkers in dementia with Lewy bodies and Parkinson’s disease dementia. Int J Geriatr Psychiatry. 2011;26(1):100-105. doi:10.1002/gps.2496.
4. McKeith IG, Dickson DW, Lowe J, Emre M, et al. Consortium on DLB. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium [published correction appears in Neurology. 2005;65(12):1992]. Neurology. 2005;65(12):1863-1872.
5. Allan LM, Ballard CG, Allen J, et al. Autonomic dysfunction in dementia. J Neurol Neurosurg Psychiatry. 2007;78(7):671-677.
6. Walter BL. Cardiovascular autonomic dysfunction in patients with movement disorders. Cleve Clin J Med. 2008;75(suppl 2):S54-S58.
7. Ziemssen T, Reichmann H. Treatment of dysautonomia in extrapyramidal disorders. Ther Adv Neurol Disord. 2010;3(1):53-67.
8. Thaisetthawatkul P, Boeve BF, Benarroch EE, et al. Autonomic dysfunction in dementia with Lewy bodies. Neurology. 2004;62(10):1804-1809.
9. Horimoto Y, Matsumoto M, Nakazawa H, et al. Cognitive conditions of pathologically confirmed dementia with Lewy bodies and Parkinson’s disease with dementia. J Neurol Sci. 2003;216(1):105-108.
10. Magerkurth C, Schnitzer R, Braune S. Symptoms of autonomic failure in Parkinson’s disease: prevalence and impact on daily life. Clin Auton Res. 2005;5(2):76-82.
11. Jellinger KA. A critical reappraisal of current staging of Lewy-related pathology in human brain. Acta Neuropathol. 2008;116(1):1-16.
12. Pathak A, Senard JM. Pharmacology of orthostatic hypotension in Parkinson’s disease: from pathophysiology to management. Expert Rev Cardiovasc Ther. 2004;2(3):393-403.
13. Fornai F, Ruffoli R, Soldani P, Ruggieri S, Paparelli A. The “Parkinsonian heart”: from novel vistas to advanced therapeutic approaches in Parkinson’s disease. Curr Med Chem. 2007;14(23):2421-2428.
14. Courbon F, Brefel-Courbon C, Thalamas C, et al. Cardiac MIBG scintigraphy is a sensitive tool for detecting cardiac sympathetic denervation in Parkinson’s disease. Mov Disord. 2003;18(8):890-897.
15. Fujishiro H, Frigerio R, Burnett M, et al. Cardiac sympathetic denervation correlates with clinical and pathologic stages of Parkinson’s disease. Mov Disord. 2008;23(8):1085-1092.
16. Mitsui J, Saito Y, Momose T, et al. Pathology of the sympathetic nervous system corresponding to the decreased cardiac uptake in 123I-metaiodobenzylguanidine (MIBG) scintigraphy in a patient with Parkinson disease. J Neurol Sci. 2006;243(1-2):101-104.
17. Saiki S, Hirose G, Sakai K, et al. Cardiac 123I-MIBG scintigraphy can assess the disease severity and phenotype of PD. J Neurol Sci. 2004;220(1-2):105-111.
18. Shibata M, Morita Y, Shimizu T, Takahashi K, Suzuki N. Cardiac parasympathetic dysfunction concurrent with cardiac sympathetic denervation in Parkinson’s disease. J Neurol Sci. 2009;276(1-2):79-83.
19. Takatsu H, Nishida H, Matsuo H, et al. Cardiac sympathetic denervation from the early stage of Parkinson’s disease: clinical and experimental studies with radiolabeled MIBG. J Nucl Med. 2000;41(1):71-77.
20. Taki J, Nakajima K, Hwang EH, et al. Peripheral sympathetic dysfunction in patients with Parkinson’s disease without autonomic failure is heart selective and disease specific. Eur J Nucl Med. 2000;27(5):566-573.
21. Hamada K, Hirayama M, Watanabe H, et al. Onset age and severity of motor impairment are associated with reduction of myocardial 123I-MIBG uptake in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2003;74(4):423-426.
22. Matsui H, Nishinaka K, Oda M, Komatsu K, Kubori T, Udaka F. Does cardiac metaiodobenzylguanidine (MIBG) uptake in Parkinson’s disease correlate with major autonomic symptoms? Parkinsonism Relat Disord. 2006;12(5):284-288.
23. Miller VM, Kenny RA, Slade JY, Oakley AE, Kalaria RN. Medullary autonomic pathology in carotid sinus hypersensitivity. Neuropathol Appl Neurobiol. 2008;34(4):403-411.
24. Sclater A, Alagiakrishnan K. Orthostatic hypotension. A primary care primer for assessment and treatment. Geriatrics. 2004;59(8):22-27.
25. Andersson M, Hansson O, Minthon L, Ballard CG, Londos E. The period of hypotension following orthostatic challenge is prolonged in dementia with Lewy bodies. Int J Geriatr Psychiatry. 2008;23(2):192-198.
26. Low PA. Prevalence of orthostatic hypotension. Clin Auton Res. 2008;18(suppl 1):8-13.
27. Biaggioni I. Parkinson’s disease: autonomic neuronopathy with impaired cardiovascular regulation. Hypertension. 2007;49(1):21-22.
28. Allcock LM, Ullyart K, Kenny RA, Burn DJ. Frequency of orthostatic hypotension in a community based cohort of patients with Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2004;75(10):1470-1471.
29. Senard JM, Raï S, Lapeyre-Mestre M, Brefel C, Rascol O, Rascol A, et al. Prevalence of orthostatic hypotension in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1997;63(5):584-589.
30. Wüllner U, Schmitz-Hübsch T, Antony G, Fimmers R, Spottke A, Oertel WH, et al. KNP e.V. Autonomic dysfunction in 3414 Parkinson’s disease patients enrolled in the German Network on Parkinson’s disease (KNP e.V.): the effect of ageing. Eur J Neurol. 2007;14(12):1405-1408.
31. Allcock LM, Kenny RA, Mosimann UP, et al. Orthostatic hypotension in Parkinson’s disease: association with cognitive decline? Int J Geriatr Psychiatry. 2006;21(8):778-783.
32. Eigenbrodt ML, Rose KM, Couper DJ, Arnett DK, Smith R, Jones D. Orthostatic hypotension as a risk factor for stroke: the atherosclerosis risk in communities (ARIC) study, 1987-1996. Stroke. 2000;31(10):2307-2313.
33. Elmstáhl S, Rosén I. Postural hypotension and EEG variables predict cognitive decline: results from a 5-year follow-up of healthy elderly women. Dement Geriatr Cogn Disord. 1997;8(3):180-187.
34. Rose KM, Eigenbrodt ML, Biga RL, et al. Orthostatic hypotension predicts mortality in middle-aged adults: the Atherosclerosis Risk In Communities (ARIC) Study. Circulation. 2006;114(7):630-636.
35. Verwoert GC, Mattace-Raso FU, Hofman A, et al. Orthostatic hypotension and risk of cardiovascular disease in elderly people: the Rotterdam study. J Am Geriatr Soc. 2008;56(10):1816-1820.
36. Kenny RA, Kalaria R, Ballard C. Neurocardiovascular instability in cognitive impairment and dementia. Ann N Y Acad Sci. 2002;977:183-195.
37. Mussi C, Ungar A, Salvioli G, et al. Evaluation of Guidelines in Syncope Study 2 Group. Orthostatic hypotension as cause of syncope in patients older than 65 years admitted to emergency departments for transient loss of consciousness. J Gerontol A Biol Sci Med Sci. 2009;64(7):801-806.
38. Matinolli M, Korpelainen JT, Korpelainen R, Sotaniemi KA, Myllylä VV. Orthostatic hypotension, balance and falls in Parkinson’s disease. Mov Disord. 2009;24(5):745-751.
39. Allan LM, Ballard CG, Rowan EN, Kenny RA. Incidence and prediction of falls in dementia: a prospective study in older people. PLoS One. 2009;4(5):e5521.
40. Haensch CA, Lerch H, Jörg J, Isenmann S. Cardiac denervation occurs independent of orthostatic hypotension and impaired heart rate variability in Parkinson’s disease. Parkinsonism Relat Disord. 2009;15(2):134-137.
41. Jamnadas-Khoda J, Koshy S, Mathias CJ, Muthane UB, Ragothaman M, Dodaballapur SK. Are current recommendations to diagnose orthostatic hypotension in Parkinson’s disease satisfactory? Mov Disord. 2009;24(12):1747-1751.
42. Peralta C, Stampfer-Kountchev M, Karner E, et al. Orthostatic hypotension and attention in Parkinson’s disease with and without dementia. J Neural Transm. 2007;114(5):585-588.
43. Barbic F, Perego F, Canesi M, et al. Early abnormalities of vascular and cardiac autonomic control in Parkinson’s disease without orthostatic hypotension. Hypertension. 2007;49(1):120-126.
44. Senard JM, Brefel-Courbon C, Rascol O, Montastruc JL. Orthostatic hypotension in patients with Parkinson’s disease: pathophysiology and management. Drugs Aging. 2001;18(7):495-505.
45. Schoffer KL, Henderson RD, O’Maley K, O’Sullivan JD. Nonpharmacological treatment, fludrocortisone, and domperidone for orthostatic hypotension in Parkinson’s disease. Mov Disord. 2007;22(11):1543-1549.
46. Mosnaim AD, Abiola R, Wolf ME, Perlmuter LC. Etiology and risk factors for developing orthostatic hypotension. Am J Ther. 2009;17(1):86-91.
47. Poon IO, Braun U. High prevalence of orthostatic hypotension and its correlation with potentially causative medications among elderly veterans. J Clin Pharm Ther. 2005;30(2):173-178.
48. Calne DB, Brennan J, Spiers AS, Stern GM. Hypotension caused by L-dopa. Br Med J. 1970;1(5694):474-475.
49. Ziemssen T, Reichmann H. Cardiovascular autonomic dysfunction in Parkinson’s disease. J Neurol Sci. 2010;289(1-2):74-80.
50. Reichmann H, Ziemssen T. Treatment strategies for nonmotor manifestations of Parkinson’s disease. Expert Opin Pharmacother. 2009;10(5):773-784.
51. Goldstein DS, Pechnik S, Holmes C, Eldadah B, Sharabi Y. Association between supine hypertension and orthostatic hypotension in autonomic failure [published correction appears in Hypertension. 2003;43(4):e12]. Hypertension. 2003;42(2):136-142.
52. Goldstein DS, Holmes CS, Dendi R, Bruce SR, Li ST. Orthostatic hypotension from sympathetic denervation in Parkinson’s disease. Neurology. 2002;58(8):1247-1255.
53. Goldstein DS, Eldadah BA, Holmes C, et al. Neurocirculatory abnormalities in Parkinson disease with orthostatic hypotension: independence from levodopa treatment. Hypertension. 2005;46(6):1333-1339.
54. Biaggioni I, Robertson RM. Hypertension in orthostatic hypotension and autonomic dysfunction. Cardiol Clin. 2002;20(2):291-301, vii.
55. Rascol O, Goetz C, Koller W, Poewe W, Sampaio C. Treatment interventions for Parkinson’s disease: an evidence based assessment. Lancet. 2002;359(9317):1589-1598.
56. Pathak A, Senard JM. Blood pressure disorders during Parkinson’s disease: epidemiology, pathophysiology and management. Expert Rev Neurother. 2006;6(8):1173-1180.
57. Coelho M, Ferreira J, Rosa M, Sampaio C. Treatment options for non-motor symptoms in late-stage Parkinson’s disease. Expert Opin Pharmacother. 2008;9(4):523-535.
58. Senard, JM. Blood pressure disorders during idiopathic Parkinson’s disease [in French]. Presse Med. 2003;32(26):1231-1237.
59. Singer W, Sandroni P, Opfer-Gehrking TL, et al. Pyridostigmine treatment trial in neurogenic orthostatic hypotension. Arch Neurol. 2006;63(4):513-518.
60. Gales BJ, Gales MA. Pyridostigmine in the treatment of orthostatic intolerance. Ann Pharmacother. 2007;41(2):314-318.


