Rectovesical Fistula in a 7-Year-Old Boy
A 7-year-old boy with a past history of recurrent urinary tract infections (UTIs) presented to the emergency department with gross hematuria, dysuria, and UTI resistant to treatment with oral antibiotics.
His past medical history was relevant for anorectal impalement. Five years before this admission, he had landed on a pencil while jumping on a bed (the pencil was hidden between 2 twin beds); the patient had undergone suturing for perineal laceration. Two months after the trauma, he was diagnosed with a UTI. A scout film of the abdomen revealed a bladder stone. Renal ultrasonography was normal, while a voiding cystourethrogram (VCUG) and a computed tomography (CT) scan showed a large bladder calculus.
During anterior open cystolithotomy, a 5-cm stone was removed; also removes were a foreign body described as umbilical tape, hemorrhagic rubbery tissue, and surrounding inflammatory granulation tissue. Postsurgical VCUG showed grade 1 right vesicoureteral reflux; no fistula was described. The subsequent 4 years of follow-up revealed at least 10 chronic, recurrent UTIs documented with urine culture (mostly with Escherichia coli as the causative agent), with repeated VCUGs showing no fistula or reflux.
Routine review of systems during this hospitalization revealed persistent passage of gas from the urethra toward the completion of urination, which had been noticed 3 weeks before admission. The boy denied fecaluria.
Physical examination revealed an afebrile patient. He was a Tanner stage I, uncircumcised male with testes descended. His weight was 32.1 kg, height was 125 cm, and blood pressure was normal. He was normocephalic, with no gross head deformities. The abdomen was benign with no costovertebral angle tenderness and no suprapubic tenderness. A perianal scar of less than 1 cm in diameter was noted. No edema was present.
Laboratory urinalysis results were as follows: pH was 5.0; specific gravity was 1.026; hemoglobin was moderate; nitrite was negative; leukocyte esterase was small in amount; leukocyte count was 30 to 50 per high-power field; erythrocyte count was 15 to 30 per high-power field; mucous was moderate; epithelial cell count was 1 to 10 per low-power field. Urine culture grew more than 100,000 colony-forming units/mL of Morganella morganii.
Complete blood count with differential showed a leukocyte count of 7,100/µL; segmented neutrophils were 57.8%, and lymphocytes were 33.8%, with no band neutrophils detected. On chemistry panel, blood urea nitrogen was 4 mg/dL, creatinine was 0.4 mg/dL, and carbon dioxide was 23 mEq/L. Urine cytology was negative for malignant cells, with blood cells reported.
Plain radiographs of the abdomen and a renal ultrasonography were normal. Bladder ultrasonography showed a 1.3-cm calcified focus at the base of the bladder. VCUG showed a fistulous connection between the bladder and the rectum arising from the posterior base of the bladder (Figure). CT scanning with contrast showed a heavily calcified, mushroom-like lesion with a stalk; radiocontrast agent had leaked from the colon into the bladder. Cystoscopy showed a bladder stone that was fixed to a colovesical fistula (CVF) posterior to the trigone in the base of the bladder, no urethral fistula, and a high rectal fistula 6 to 7 cm from the anus.
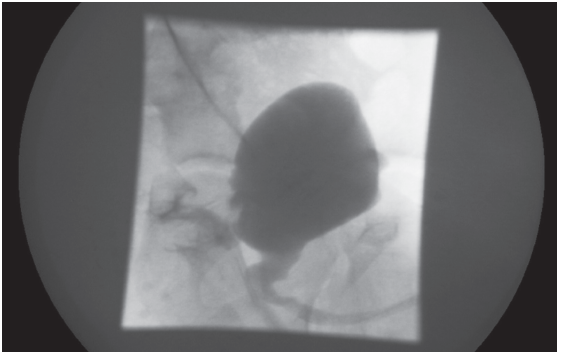
Figure – VCUG showed a rectovesicular fistula.
The diagnosis was a rectovesical fistula, presenting as pneumaturia, with a history of perineal trauma, chronic UTIs, and recurrent bladder calculus. The patient responded well to intravenous antibiotics, and subsequent urine cultures were negative. He was discharged on oral antibiotics with a plan for definitive surgical repair.
PNEUMATURIA: AN OVERVIEW
Pneumaturia is the passage of gas in the urine, which is an uncommon symptom in the pediatric population.1 Pneumaturia most commonly is reported in older adults as a symptom of diverticulitis or malignancy. In children, however, pneumaturia most often is associated with trauma. Very few cases have been reported in the literature in which anorectal impalement in a child was associated with bladder perforation.2,3 None of these cases featured pneumaturia as the presenting sign leading to the diagnosis of enterovesical fistula.
Rectal injuries in the pediatric population are uncommon and usually result from sexual assault, iatrogenic causes (eg, use of thermometers or enemas), motor vehicle accidents, or accidental falls.2,4 In most of the reported pediatric cases of rectal injury, impalement involving foreign body trauma to the anus or rectum is most common.2,4,5 Depending on how the impalement has occurred, it is possible to predict the organs likely to be involved. The foreign body can enter the anorectal canal directly, or it may pass through the perineum or buttocks first, as was the case in this patient. If the trauma is directed posteriorly, then retrorectal, retroperitoneal, and vascular injuries must be considered. If the trauma is directed anteriorly, then peritoneal perforation should be foreseen at the pouch of Douglas, with injury of the uterus in girls or lesion of the bladder and bowel in boys.4 The most common secondary injury after a rectal penetrating injury is to the genitourinary system, with or without peritoneal involvement.3 Many investigators emphasize that a lack of external physical findings does not rule out further intra-abdominal lesions, hence the importance of maintaining a high index of suspicion.2,4
Diagnostic testing. Radiologic diagnosis of CVF is notoriously difficult, and no single radiologic investigation has been found to be satisfactory in defining the fistulous tract.6,7 Plain radiographs of the abdomen have been used, but they can fail to show free air or bladder air-fluid levels.8 While VCUG is central to the study of fistulas of the lower urinary tract, it has proven positive in just over 33% of cases.9 CT scans are useful for diagnosis and are considered the primary test in some cases, although they often fail to demonstrate the fistulous tract. On the other hand, CT scans can show intravesical air, focal bladder-wall thickening, and extraluminal masses, and they can define the surrounding soft-tissue structures,8,10,11 which can help support the diagnosis. Cystoscopy is suggestive of enterovesical fistula in almost all cases but fails to demonstrate the fistula in approximately half of them.7,10 Cystoscopy findings compatible with enterovesical fistula include a papillary, tumor-like appearance due to bullous edema around the fistulous opening, or erythema and mucus-like substance over the wall of the bladder due to chronic inflammation and calcification.9 Barium enema studies demonstrate only 20% to 50% of fistulas7; upper gastrointestinal series are not beneficial. Magnetic resonance imaging and ultrasonography have been used to delineate enterovesical fistulas, although experience with them is limited.10 While rectosigmoidoscopy is recommended in some cases, it has shown fistulous tracts in only 16% of cases.5,11
Etiology and symptoms. In adults, most enterovesical fistulas are between the bladder and the sigmoid colon.6 The etiology includes diverticulosis, prior surgery, malignancy, and Crohn disease.7,8,12 Symptoms include UTIs, recurrent cystitis, terminal pneumaturia (a pathognomonic finding), fecaluria, fever, and abdominal pain.6,10-12
Enterovesical fistulas in children almost always are due to surgery or trauma. This patient presented with recurrent cystitis and pyelonephritis, abdominal pain, and late pneumaturia (3 weeks before admission). This would be the first reported case of late pneumaturia secondary to enterovesical fistula in a child.
TAKE-HOME MESSAGE
Pediatric anorectal impalement associated with pneumaturia late in the course of CVF has not been reported in the literature before now.
Pneumaturia and fecaluria should be included routinely in the review of systems in patients with recurrent UTIs, especially in patients with prior abdominal surgery, perineal trauma, or calculus. In cases of impalement, the physical findings do not always correlate with the presence of internal lesions. Thus, a high index of suspicion for CVF and further workup to clarify the presence and exact location of the CVF are important.
REFERENCES:
1. Cohen N, Modai D, Golik A, et al. Pneumaturia: need for diagnostic alertness. Isr J Med Sci. 1986;
22(2):123-126.
2. Kim S, Linden B, Cendron M, Puder M. Pediatric anorectal impalement with bladder rupture: case
report and review of the literature. J Pediatr Surg. 2006;41(9):E1-E3.
3. Weber S, Mauch W, Kalayoglu M, Moon TD. Intraperitoneal and extraperitoneal bladder rupture secondary to rectal impalement. J Trauma. 1995;38(5):818-819.
4. Bronkhorst MW, Wilde JC, Hamming JF, Heij HA. Anorectal impalement in a pediatric patient with transanal evisceration of small bowel. J Pediatr Surg. 2007;42(9):E23-E25.
5. Beiler HA, Zachariou Z, Daum R. Impalement and anorectal injuries in childhood: a retrospective study of 12 cases. J Pediatr Surg. 1998;33(8):1287-1291.
6. Rodrigo E, Ruiz JC, López-Rasines G, et al. Recurrent graft pyelonephritis and pneumaturia resulting from a colovesical fistula secondary to silent diverticulitis. Nephrol Dial Transplant. 1998;13(4):1001-1003.
7. Garcea G, Majid I, Sutton CD, Pattenden CJ, Thomas WM. Diagnosis and management of colovesical fistulae: six-year experience of 90 consecutive cases. Colorectal Dis. 2006;8(4):347-352.
8. Raymond PL, Gibler WB. Detection of colovesical fistula in the emergency department: report of a case. Am J Emerg Med. 1989;7(2):191-195.
9. Chitale SV, Choudhury A, Gaches CG. Transurethral fistulography—a useful technique in investigating recurrent undiagnosed pneumaturia. World J Urol. 2001;19(4):259-260.
10. Yu NC, Raman SS, Patel M, Barbaric Z. Fistulas of the genitourinary tract: a radiologic review.
Radiographics. 2004;24(5):1331-1352.
11. Najjar SF, Jamal MK, Savas JF, Miller TA. The spectrum of colovesical fistula and diagnostic paradigm. Am J Surg. 2004;188(5):617-621.
12. Puyol M, Alcaraz A, Romero JA, et al. Entero-urinary fistula. A study of 22 cases. [in Spanish]. Arch Esp Urol. 1990;43(5):457-460.


