HISTORY
Twenty-two-month-old girl seen in the emergency department (ED) after several hours of abdominal pain associated with non-bloody, non-bilious emesis. Over past 2 months, has had 7 or 8 similar episodes of abdominal pain followed by emesis 1 to 2 hours later.
Pain appeared severe, causing patient to cease all activity, clutch her epigastric region, cry, and remain in the fetal position. Mother unable to describe quality of pain or whether it radiated.
_________________________________________________________________________________________________________________________________________
Related Content
A Collection of Hernias and Hernia-Associated Syndromes
Diaphragmatic Hernia
_________________________________________________________________________________________________________________________________________
During all episodes, pain began suddenly and then continued intermittently for several hours. Emesis alleviated the pain. Temporal relationship of the start of abdominal pain to oral intake was not clear because episodes occurred at different times throughout the day. The patient's mother thought the intensity of pain was increasing. Between episodes, patient was playful, energetic, had good oral intake, and a varied diet. However, the mother noted some weight loss during the past month.
 Her pediatrician, seen multiple times since the start of these episodes, had diagnosed gastritis. The patient had made 3 ED visits; laboratory study results were normal. Bowel intussusception was suspected but abdominal ultrasonographic findings were normal.
Her pediatrician, seen multiple times since the start of these episodes, had diagnosed gastritis. The patient had made 3 ED visits; laboratory study results were normal. Bowel intussusception was suspected but abdominal ultrasonographic findings were normal.
No history of sick contacts, trauma, or chemical ingestion. Patient's bowel habits normal; adequate urinary output. Immunizations up-to-date. No drug or food allergies. Developmentally appropriate.
Family history not contributory. Patient lived with her mother, father, 4 siblings, and dog. Six months before current episode, patient was taken to Mexico for 1 month. No other recent travel. Patient did not attend day care.
Past medical history relatively unremarkable. Born at 36 weeks' gestation via "repeat" cesarean section. Birth weight, 1820 g with moderate intrauterine growth restriction. Antenatal maternal α-fetoprotein (AFP) level said to be elevated, but further evaluation for fetal anomaly was not carried out. Patient admitted to neonatal ICU for 10 days after delivery because of poor oral intake. There were no respiratory problems during neonatal hospitalization.
At age 20 months, incontinentia pigmenti was diagnosed based on hypopigmented lesions on patient's extremities, which had been present since 2 months of age.
PHYSICAL EXAMINATION
Notably thin. Weight, 8.3 kg (less than the third percentile); height, 83 cm (25th to 50th percentile). Afebrile; vital signs normal. Lungs clear. Regular heart rate and rhythm; no murmurs. Soft and non-distended abdomen; discomfort on palpation when the patient was awake but not while sleeping. No rebound tenderness or guarding. Bowel sounds decreased in the abdomen. Skin showed hypopigmented linear lesions along the lines of Blaschko on upper and lower extremities.
LABORATORY RESULTS
 Complete metabolic panel and pancreatic enzyme levels all within normal limits. Normal white blood cell count and differential. Low hemoglobin (9.3 g/dL) and hematocrit (29.9%). Patient hospitalized for further evaluation.
Complete metabolic panel and pancreatic enzyme levels all within normal limits. Normal white blood cell count and differential. Low hemoglobin (9.3 g/dL) and hematocrit (29.9%). Patient hospitalized for further evaluation.
IMAGING STUDY RESULTS
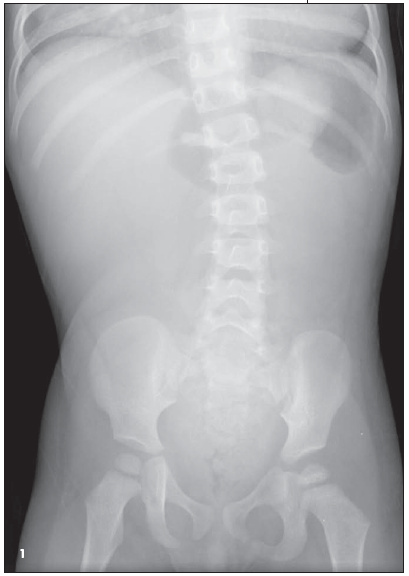
Intussusception ruled out by ultrasonography. Abdominal flat plate radiographs-obtained to evaluate for possible intermittent volvulus and bowel obstruction- showed tubular radiolucencies in the upper abdomen extending to the chest toward the midline. Decreased gas noted in lower abdomen (Figure 1). Chest fluoroscopy showed several rounded lucencies in the midlineof the chest on right and left sites, with a tubular structure passing over the gastroesophageal junction. An enlarged cardiac silhouette was also noted (Figure 2).
WHAT'S YOUR DIAGNOSIS?
Answer on next page
ANSWER: DIAPHRAGMATIC HERNIA
There are 3 types of diaphragmatic hernias: Bochdalek, Morgagni, and hiatal. They differ with respect to frequency of occurrence, timing of clinical manifestations, clinical significance, and outcome.
Bochdalek hernia. From the fourth to sixth week of embryonic life, there is a complete partition between the primitive thoracic and abdominal cavities. Subsequently, formation of the periphery of the diaphragm continues up to the 12th week of intrauterine life. The diaphragm forms by the fusion of the septum transversum, the pleuroperitoneal membranes, and the dorsal mesentery of the esophagus, with a later contribution from the body wall. Hernia through the foramen of Bochdalek is a posterolateral diaphragmatic hernia. The posterolateral defects result from defective formation and/or fusion of the pleuroperitoneal membrane. If the fusion has not occurred by the 10th week, when the intestines return to the abdomen from the umbilical cord, or if the bowel returns prematurely, herniation into the chest can occur.1
This long-accepted mechanism for congenital diaphragmatic hernia has been questioned, however, and alternative pathophysiology-such as problems arising from the pleuroperitoneal fold and/or primary defect in muscular development of the diaphragm-has been proposed. 2,3 An overwhelming majority (88%) of the posterolateral or foramen of Bochdalek hernias are left-sided. 4,5 This may be caused by earlier closure of the right pleuroperitoneal opening. Posterolateral defects vary in size from small triangular or oval defects with well-developed diaphragmatic muscle anteriorly and a rim of muscle posteriorly. The content of the chest varies; the left side of the pleural cavity usually contains stomach, small intestines, colon, spleen, and the left lobe of liver. A hernia on the right side contains liver and variable amounts of small and large intestines.1
Morgagni hernias. These are uncommon (they account for only 2% of congenital diaphragmatic hernias) in neonates.6 The foramen of Morgagni is a gap, or a potential gap, lying between the sternal and costal attachments of the diaphragm. Herniation through the foramen of Morgagni is also referred to as parasternal. These hernias are always covered by a membranous sac while a membranous sac is noted only in 20% of cases of Bochdalek hernias. Those that manifest during the neonatal period are more frequently associated with other congenital anomalies, particularly cardiac anomalies.1
Because of its anterior location, the Morgagni hernia usually involves the colon or small intestine and can present later in life with GI symptoms. Only 10% to 20% of Morgagni-type hernias manifest during the neonatal period with symptoms of emesis or mild respiratory distress. Patients are generally asymptomatic and discovery typically results from diagnostic imaging undertaken for unrelated reasons.5 Air-filled viscera in the mediastinum on plain chest radiographs-as seen in our patient-is the simplest diagnostic tool to detect these hernias. It is uncertain whether our patient's hernia was acquired or congenital. Because of the increased maternal serum AFP level, moderate intrauterine growth restriction, and lack of postnatal catch-up growth, there is a high likelihood that it was congenital.
Hiatal hernia. A hiatal hernia occurs when a portion of the stomach protrudes into the chest cavity through the diaphragmatic esophageal hiatus. There are 2 types of hiatal hernias:
•In the sliding type (the most common), the stomach and the section of the esophagus that joins the stomach slide up into the chest through the hiatus.
•In the paraesophageal type, part of the stomach protrudes through the esophageal hiatus. This type of hiatal hernia is rare, but it has more pathological significance because the herniated portion of the stomach may strangulate.
Hiatal hernias are more common in adults secondary to muscle weakness, trauma, obesity, postoperative changes, or increased abdominal pressure (eg, during pregnancy or with chronic coughing). They are commonly associated with gastroesophageal reflux disease (GERD).7
COMMENT
Congenital diaphragmatic hernia (CDH) is most often a posterolateral defect in the left hemidiaphram (88%) on one side, which leads to herniation of the abdominal viscera into the thorax, resulting in pulmonary hypoplasia and respiratory embarrassment after birth.1,4-6 CDH occurs in approximately 1 in 2500 to 5000 live births and as frequently as 1 in 2200 prenatal ultrasound studies.4 Female infants are affected twice as often as males.5 The cause of CDH is not known, but genetic factors may play a role because familial cases have been reported. 8 CDH is frequently associated with pulmonary hypoplasia; other associated problems are malrotation of the midgut, cardiac and central nervous systems, and chromosomal abnormalities.9 Associated congenital abnormalities are much more frequent and severe in stillborn fetuses with CDH.4
The diagnosis of CDH is commonly made by antenatal ultrasonography as early as 16 to 24 weeks' gestation. In one study, the antenatal diagnosis was reported in 93% of patients with CDH.10 Typical ultrasonographic findings in the fetus with CDH include an echogenic chest mass, bowel loops within the chest, polyhydramnios, absent or intrathoracic gastric bubble, mediastinal shift to the contralateral side, intrathoracic liver, and fetal hydrops.10 The differential diagnosis includes type 1 congenital cystic adenomatoid malformation; bronchogenic cysts; neuroenteric cysts; and cystic mediastinal teratoma, which may mimic the appearance of herniated bowel. Identification of abnormal upper abdominal anatomy and presence of peristalsis in herniated bowel loops helps distinguish CDH from other diagnoses.4
CDH should be suspected in a newborn with scaphoid abdomen and respiratory distress (secondary to the abdominal viscera being in the chest) and pulmonary hypoplasia-particularly on the ipsilateral side. When CDH is diagnosed antenatally or suspected at birth, symptomatic infants requiring assisted ventilation should be endotracheally intubated and gently ventilated while a nasogastric tube is inserted for intermittent suction. Findings of a pneumothorax or mediastinal shift on chest radiograph or a gasless abdomen on abdominal films may also point to the diagnosis.
Once CDH is diagnosed, an echocardiogram should be obtained to evaluate for pulmonary hypertension and for associated cardiac anomalies, such as atrial and ventral septal defects or hypoplastic left heart syndrome. Such cardiac anomalies occur in up to 25% of cases of CDH. Other anomalies found in CDH are esophageal atresia, omphalocele, genitourinary anomalies, neural tube defects, cardiovascular defects, and hydrocephalus.5
Surgical repair was once considered emergent at the time of birth; however survival rates for early surgery were about 50%.4 Infants with severe pulmonary hypertension or pulmonary hypoplasia had the poorest prognosis. Stabilization with assisted mechanical ventilation, inotropic agents, and gastric decompression, as well as resolution of persistent pulmonary hypertension with nitric oxide and/or extracorporeal membrane oxygenation improves survival rates to 70% to 90%.4-6 In utero surgical repair of the left CDH was described by Harrison and colleagues,11 but the outcome was no better than with postnatal surgical repair. Similarly, in utero occlusion of the fetal trachea with a clip procedure to enhance fetal lung growth, increase alveolar numbers and alveolar surface area, and reduce herniated abdominal viscera did not improve the outcome.12
 Surgical repair of CDH can be performed by an open or laparoscopic approach, with closure via a patch or sutures alone. Recurrence and infection rates are higher with patch repair. However, sutures can increase the tension on the chest wall, which may result in pectus excavatum or scoliosis. Other sequelae of CDH repair include continued pulmonary hypertension, chronic lung disease, GERD, failure to thrive, and developmental delay.4-6
Surgical repair of CDH can be performed by an open or laparoscopic approach, with closure via a patch or sutures alone. Recurrence and infection rates are higher with patch repair. However, sutures can increase the tension on the chest wall, which may result in pectus excavatum or scoliosis. Other sequelae of CDH repair include continued pulmonary hypertension, chronic lung disease, GERD, failure to thrive, and developmental delay.4-6
Our patient underwent reduction of a large incarcerated Morgagni-type hernia that involved the cecum and distal ileum, with suture closure of the defect in the diaphragm. There were no postoperative complications. Postoperative chest films showed closure of the diaphragmatic defect and clear lung fields (Figure 3). The patient tolerated a regular diet and she was discharged home by the fourth day after surgery in good condition. She has had regular follow-up evaluations in our pediatric clinic. At 3¹⁄₂ years of age, the child is healthy but remains at lower percentile for height and weight.
 Surgical repair of CDH can be performed by an open or laparoscopic approach, with closure via a patch or sutures alone. Recurrence and infection rates are higher with patch repair. However, sutures can increase the tension on the chest wall, which may result in pectus excavatum or scoliosis. Other sequelae of CDH repair include continued pulmonary hypertension, chronic lung disease, GERD, failure to thrive, and developmental delay.4-6
Surgical repair of CDH can be performed by an open or laparoscopic approach, with closure via a patch or sutures alone. Recurrence and infection rates are higher with patch repair. However, sutures can increase the tension on the chest wall, which may result in pectus excavatum or scoliosis. Other sequelae of CDH repair include continued pulmonary hypertension, chronic lung disease, GERD, failure to thrive, and developmental delay.4-6


 Her pediatrician, seen multiple times since the start of these episodes, had diagnosed gastritis. The patient had made 3 ED visits; laboratory study results were normal. Bowel intussusception was suspected but abdominal ultrasonographic findings were normal.
Her pediatrician, seen multiple times since the start of these episodes, had diagnosed gastritis. The patient had made 3 ED visits; laboratory study results were normal. Bowel intussusception was suspected but abdominal ultrasonographic findings were normal. Complete metabolic panel and pancreatic enzyme levels all within normal limits. Normal white blood cell count and differential. Low hemoglobin (9.3 g/dL) and hematocrit (29.9%). Patient hospitalized for further evaluation.
Complete metabolic panel and pancreatic enzyme levels all within normal limits. Normal white blood cell count and differential. Low hemoglobin (9.3 g/dL) and hematocrit (29.9%). Patient hospitalized for further evaluation.