Severe Methicillin-Resistant Staphylococcus Aureus Septicemia in a Case of Misdiagnosed Crusted (Norwegian) Scabies
Crusted (Norwegian) scabies is caused by the itch mite Sarcoptes scabiei var hominis. It is a highly contagious variant of classic scabies that affects elderly patients in particular,1 especially residents of nursing homes and individuals who are immunocompromised, malnourished, or cognitively impaired.2,3 Crusted scabies often presents atypically from classic scabies, making it a challenging clinical diagnosis to reach, as it may mimic a host of other dermatoses.4 A delayed or missed diagnosis of crusted scabies may result in serious morbidity, including severe secondary infection with life-threatening methicillin-resistant Staphylococcus aureus (MRSA) via skin eruptions, and epidemic infestations in hospitals and nursing homes.4-6
We report a case of crusted scabies in an elderly nursing home resident. The condition went misdiagnosed as drug-induced dermatitis for >2 months. As a result, the patient experienced terminal complications from MRSA sepsis and there was an outbreak of crusted scabies among several other nursing home residents and staff members. The case presentation illustrates how failure to diagnose and eliminate these microscopic, burrowing parasites promptly can have life-threatening consequences, particularly for elderly and immunocompromised individuals.
Case Presentation
A 73-year-old male nursing home resident was transferred from a regional long-term acute care (LTAC) facility to a university hospital because of persistent MRSA sepsis—despite having received intravenous (IV) antibiotics for almost 2 weeks—and for worsening dyspnea. He was previously admitted to a large community medical center for 6 days for a worsening chronic rash, which was first noticed approximately 8 weeks prior to his current presentation at the university hospital. Drug-induced dermatitis was diagnosed at the community medical center, and his home medications were discontinued. On initial blood tests, the patient was also found to have MRSA bacteremia of unclear origin and was started on IV antibiotics (linezolid). After 4 days on IV antibiotics, he was discharged to the LTAC facility for continued antibiotic treatment. During his 8-day stay at the LTAC facility, his general medical condition worsened and he developed diminished mentation from baseline, acute-on-chronic renal insufficiency, shortness of breath, and metabolic acidosis.
Upon transfer to the university hospital, his accompanying medical records revealed a medical history significant for mild cognitive impairment, a status of class III morbid obesity (body mass index, >40 kg/m2) post-gastric bypass surgery, obstructive sleep apnea treated with bilevel positive airway pressure (BiPAP), chronic obstructive pulmonary disease, hypertension, hypothyroidism, chronic kidney disease, osteoarthritis, and poorly controlled diabetes mellitus. On admission, his blood pressure was 94/56 mm Hg, his heart rate was 55 beats per minute, and his respiratory rate was 21 breaths per minute. He was afebrile, with a temperature of 96.8°F, and mildly hypoxemic, with an oxygen saturation of 91% while breathing room air.
On physical examination, the patient was found to be a morbidly obese, elderly white man in mild respiratory distress. He was alert and able to obey verbal commands. Although nonverbal, he scored 11/15 on the Glasgow Coma Scale (eye opening, 4; verbal, 1; motor, 6). Dry mucus membranes were noted, and diffuse crepitations were heard bilaterally on auscultation of the lungs. S1 and S2 heart sounds were distant. On inspection, his abdomen was obese, moved with respiration, had normoactive bowel sounds on auscultation, and had no masses or tenderness on palpation. A remarkable pustular, hyperkeratotic rash with yellow flaky plaques affected his upper limbs, trunk, back, and thighs (Figures 1 and 2). The patient’s face and neck were spared. Using the “rule of fives,” which is preferentially used to estimate burned body surface area in obese burn victims,7 the patient’s physicians estimated that 88% of his body surface area was affected by rash.

Initial laboratory data included the following: a hemoglobin level of 12.1 g/dL; hematocrit of 36.5%; and a white blood cell count of 15,300/µL, with 84% neutrophils, 10% lymphocytes, and 4% eosinophils. The patient’s platelet count was 209 ×103/µL, and mean corpuscular volume was 94 fL. Urinalysis was positive for blood and leukocytes, and urine microscopy revealed yeast and bacteria. Initial chest x-ray was unremarkable, and no acute intracranial changes were observed with head computed tomography scanning. Cardiac markers were normal, with no evidence of myonecrosis, and a 12-lead electrocardiogram showed sinus bradycardia. A two-dimensional echocardiogram showed a heart rate of <60 beats per minute, with an ejection fraction of 50% to 60% and no evidence of endocarditis. After blood, urine, and sputum cultures were drawn, the patient was given IV fluids and empirically started on triple IV antibiotic therapy using clindamycin 600 mg, levofloxacin 750 mg, and linezolid 600 mg. He also received fluconazole 150 mg by mouth. BiPAP was initiated and he was admitted to the medical intensive care unit (MICU) under contact isolation for MRSA.
In the MICU, the patient was started on appropriate treatments for his other underlying conditions, including methylprednisolone 80 mg IV every 8 hours, diphenhydramine 50 mg IV every 8 hours, levothyroxine 75 mcg orally once daily, and regular insulin/D5 infusion at 125 mL/hour. He also received once-daily prophylactic treatments for deep vein thrombosis and gastrointestinal bleeding, which consisted of enoxaparin 40 mg subcutaneously and pantoprazole 40 mg orally, respectively. The aforementioned blood culture grew MRSA and Enterococcus faecalis, while the urine culture grew gram-negative bacteria. Following the results of sensitivity studies, antibiotic coverage was changed to meropenem 500 mg orally every 12 hours, vancomycin 1000 mg IV every 8 hours (with peak and trough monitoring ordered after every third dose), and micafungin 100 mg IV once daily. The infectious diseases team was consulted for optimal management.
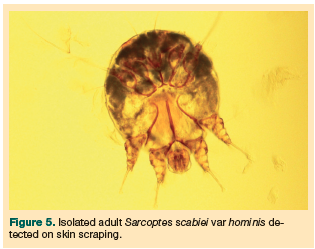
The dermatology team had been consulted on initial presentation, and within 24 hours the team had confirmed the definitive diagnosis as a heavy infestation of crusted scabies, which was confirmed following multiple skin scrapings and a skin biopsy. Skin biopsy specimens showed an abundance of mites and eggs, particularly in the thickened stratum corneum within areas of hyperkeratotic, inflamed epidermis (Figures 3-5).

 The patient was immediately started on the standard combination regimen of 5% topical permethrin, applied thoroughly to affected areas once weekly, and oral ivermectin 200 mcg/kg, with a second dose planned for 2 weeks later. Although the crusted scabies appeared to respond gradually to treatment (Figures 6 and 7), the patient’s overall clinical course continued to deteriorate rapidly. He was emergently intubated for acute hypoxemic respiratory failure on day 3 of hospitalization. Despite receiving optimal vasopressor and ventilator support and additional antimicrobial coverage, he succumbed to multiorgan failure on day 6 of hospitalization.
The patient was immediately started on the standard combination regimen of 5% topical permethrin, applied thoroughly to affected areas once weekly, and oral ivermectin 200 mcg/kg, with a second dose planned for 2 weeks later. Although the crusted scabies appeared to respond gradually to treatment (Figures 6 and 7), the patient’s overall clinical course continued to deteriorate rapidly. He was emergently intubated for acute hypoxemic respiratory failure on day 3 of hospitalization. Despite receiving optimal vasopressor and ventilator support and additional antimicrobial coverage, he succumbed to multiorgan failure on day 6 of hospitalization.
After the patient was diagnosed with crusted scabies, the university hospital infection control officer notified the patient’s nursing home caseworker; the patient’s primary care physician was also notified. The condition was subsequently diagnosed in several other nursing home residents and staff members and then treated. An epidemic outbreak was averted at the university hospital, largely because contact precautions had been taken for both MRSA and scabies.
Discussion
Human scabies is an intensely pruritic skin infestation caused by Sarcoptes scabiei var hominis.1 Scabies is typically transmitted from one person to another. Although scabies can be treated readily, it remains common, primarily due to diagnostic delays, inadequate treatment of patients and their contacts, and improper environmental control measures, all of which allow the mite to survive and spread.8 Scabies affects persons of all ages, races, and socioeconomic status.1 Elderly, debilitated persons residing in communal settings such as nursing homes are especially susceptible to infestation with either classic or crusted (Norwegian) scabies.9 Crusted scabies also occurs in patients with mental retardation, such as those with Trisomy 21 (Down syndrome).4,10 This reported association with Trisomy 21 suggests the need for additional studies to establish any possible genetic predisposition, possibly inheritable, for cogitive impairment and mental retardation.
Although crusted scabies and classic scabies are caused by the same species of mite, the parasite load on a crusted scabies host may exceed 1 million mites, compared with 5 to 15 mites per host with classic scabies.11 Skin-homing cytotoxic T cells contribute to an imbalanced inflammatory response in the dermis of crusted scabies lesional skin, which, combined with a lack of B cells, contributes to the failure of the skin’s immune system to mount an effective response. The system failure results in the uncontrolled growth of the parasite.10
Continued on next page
The presence of both eosinophilia and elevated immunoglobulin E (IgE) levels in patients with crusted scabies has been extensively reported, which in some cases only subside upon complete resolution of symptoms and confirmation by negative skin biopsy.12 According to a recent study of the immune response to Sarcoptes scabiei antigens in crusted versus classic scabies, a quantitative IgE inhibition assay identified IgE immunoreactivity of scabies mite antigens as distinct from that of house dust mite antigens.13 This distinction is potentially important when diagnosing and managing specific types of scabies. Progress in molecular biology and cloning of relevant antigens could enable the development of a diagnostic enzyme-linked immunosorbent assay (ELISA) system and candidate vaccines in the near future.14
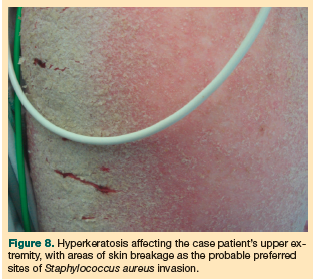 An increasing number of reports in the literature describe misdiagnosed crusted scabies in elderly patients (typically nursing home residents). The misdiagnoses almost always result in severe, life-threatening complications, most notably MRSA septicemia.15 Staphylococcus has been found to infect scabies burrows and breaks in the epidermis that are caused by scratching and the resultant excoriations in response to varying degrees of pruritus.5,15 This is the most likely route of the case patient’s MRSA infection (Figure 8).
An increasing number of reports in the literature describe misdiagnosed crusted scabies in elderly patients (typically nursing home residents). The misdiagnoses almost always result in severe, life-threatening complications, most notably MRSA septicemia.15 Staphylococcus has been found to infect scabies burrows and breaks in the epidermis that are caused by scratching and the resultant excoriations in response to varying degrees of pruritus.5,15 This is the most likely route of the case patient’s MRSA infection (Figure 8).
Many clinicians mistakenly view the absence of pruritus as a pathognomonic trait of crusted scabies, yet it is important to note that only half the patients with this condition do not report itching.3,4 In immunocompromised patients, particularly those with HIV and those receiving immunosuppressive therapy, other forms of bacterial superinfection have been observed that sometimes lead to conditions such as endocarditis or fatal brain abscesses.16-18
Transmission
The transmission of scabies is predominantly through prolonged, direct skin-to-skin contact.1 A person with mites can spread scabies even if he or she is asymptomatic.8 There may be a latent interval of ≤2 months between the primary infection, which is when the person becomes contagious, and the onset of clinical manifestations.19 Transmission by indirect contact through fomites, such as infested bedding or clothing, is less frequent, unless a person is infected with a significant number of parasites, which is the case with crusted scabies.1
Presentation
Epidemic outbreaks of scabies in industrialized countries occur primarily in institutional settings, such as prisons, long-term care (LTC) facilities, nursing homes, and hospitals.20 A typical presentation of classic scabies may be an elderly debilitated or immunocompromised individual reporting persistent, diffuse, and intense pruritus that has progressively worsened over a 2- to 3-week period. Nocturnal pruritus is highly characteristic of a scabies infestation.21 Since scabies appears to occur in clusters, a patient with scabies is likely to report at least one close contact who has the same symptoms.8
A physical examination of a patient with scabies may reveal skin lesions such as burrows, papules, pustules, nodules, and, occasionally, urticarial papules and plaques.1 These skin lesions can resemble eczema and psoriasis.9 The lesions generally affect the interdigital spaces; flexor aspects of the wrists; the axilla; the umbilicus; and the antecubital, abdominal, genital, and gluteal areas.22 In geriatric patients, scabies demonstrates a propensity for the back, often appearing as excoriations.21
Physicians should be cognizant of the crusted scabies variant, which typically infects debilitated or immunocompromised elderly patients, who may experience little or no pruritus.9 In cases of crusted scabies, mites multiply into the millions and infest the skin, causing a confluence of crusted plaques topped by thick, hyperkeratotic scales.1 These pustular lesions can very closely resemble eczema, psoriasis, or ichthyosis1 and this may be a contributing factor to the misdiagnosis of crusted scabies, especially in patients who may have a prior history of any of these dermopathies.
Diagnosis
According to the Centers for Disease Control and Prevention (CDC), skin scrapings should be used to confirm the diagnosis of scabies.8 Multiple superficial skin samples should be obtained from characteristic lesions, with the identification of mites, eggs, eggshell fragments, or scybala providing the definitive diagnosis.1 A skin biopsy may be obtained to confirm the diagnosis in atypical cases or when direct examination is not possible. On rare occasions, mites and other diagnostic features are notably absent and histological examination shows a nonspecific, delayed hypersensitivity reaction.23
Treatment
Patients given a scabies diagnosis should be treated at the time of presentation, and their close physical contacts should also receive prompt treatment.1 According to the CDC, standard therapy should include both topical and oral medications.8,24 The topical drug of choice is 5% permethrin cream; ≥2 applications, each about a week apart, may be necessary to eliminate all mites, particularly in patients with crusted (Norwegian) scabies.8 Other options for topical treatment include 10% crotamiton lotion and 1% lindane lotion, but frequent failure has been reported with crotamiton; lindane is neurotoxic and should be avoided in the elderly and in persons with a seizure disorder.21 The oral antiparasitic agent ivermectin is not approved by the US Food and Drug Administration (FDA) for the treatment of scabies, but evidence suggests that it may be safe and effective for this purpose, including for cases of crusted scabies.8 The dosage is 200 mcg/kg orally taken on an empty stomach with water; a total of ≥2 doses taken at least 7 days apart may be needed to eliminate a scabies infestation.8 Whereas immediate treatment with ivermectin is warranted in crusted scabies, in classic scabies, its use should be considered for patients who have failed treatment with, or who cannot tolerate, FDA-approved topical medications for the treatment of scabies.8,25 These CDC treatment recommendations also apply to HIV-infected patients presenting with classic scabies.24 Patients should be warned that itching may persist for as long as 4 weeks after completion of treatment. A follow-up visit should be scheduled after 1 month of treatment and should ensure that the pruritus has resolved.19
Prevention and Control
Scabies has been identified as a cause of infectious outbreaks in LTC facilities, resulting in high median case fatality rates among elderly individuals and healthcare workers.26 Early detection, treatment, and implementation of appropriate isolation and infection-control practices (eg, gloves, gowns, avoidance of direct skin-to-skin contact) are essential to prevent scabies outbreaks.8 Treatment should be provided to all household members and any of the patient’s sexual contacts, even if they are asymptomatic.19 In the event of an outbreak of scabies in a nursing home, it may be useful to treat all residents, staff, and frequent visitors, even if they are asymptomatic.9,25 One definition of an outbreak of scabies in a hospital includes only one confirmed (positive skin scraping) and at least two clinically suspected cases identified in patients, healthcare workers, volunteers, and/or visitors to the hospital during a 2-week period.27
Conclusion
The risk of severe morbidity and mortality from complications of crusted scabies highlights the importance of early diagnosis and treatment. Unfortunately, the parasitic condition is often misdiagnosed as an adverse drug reaction or as another type of dermatosis, such as psoriasis or eczema. Crusted scabies should be included in the differential diagnosis of elderly, cognitively impaired nursing home residents or immunocompromised patients who present with a persistent hyperkeratotic rash, with or without pruritus, and persistent MRSA bacteremia. Prompt diagnosis and treatment of an affected LTC resident or hospitalized patient prevents progression to severe complications and also reduces the possibility of a wider outbreak and its subsequent risks and costs.
The authors report no relevant financial relationships.
Drs. Manyindo, McDonald, Vijayan, and Adenuga are from the Department of Community and Family Medicine, and Drs. Pamireddy and Adams are from the Department of Internal Medicine, Howard University Hospital, Washington, DC.
References
1. Chosidow O. Clinical practices. Scabies. N Engl J Med. 2006;354(16):1718-1727.
2. Hopper AH, Salisbury J, Jegadeva AN, Scott B, Bennett GC. Epidemic Norwegian scabies in a geriatric unit. Age Ageing. 1990;19(2):125-127.
3. Kolar KA, Rapini RP. Crusted (Norwegian) scabies. Am Fam Physician. 1991;44(4):1317-1321.
4. Almond DS, Green CJ, Geurin DM, Evans S. Lesson of the week: Norwegian scabies misdiagnosed as an adverse drug reaction. BMJ. 2000;320(7226):35-36.
5. Shelley WB, Shelley ED, Burmeister V. Staphylococcus aureus colonisation of burrows in erythrodermic Norwegian scabies. A case study of iatrogenic contagion. J Am Acad Dermatol. 1988;19(4):673-678.
6. Buehlmann M, Beltraminelli H, Strub C, et al. Scabies outbreak in an intensive care unit with 1,659 exposed individuals—key factors for controlling the outbreak. Infect Control Hosp Epidemiol. 2009;30(4):354-360.
7. Livingston EH, Lee S. Percentage of burned body surface area determination in obese and nonobese patients. J Surg Res. 2000;91(2):106-110.
8. Parasites – scabies. Centers for Disease Control and Prevention. http://www.cdc.gov/parasites/scabies/health_professionals/index.html. Accessed August 2, 2011.
9. Weinberg JM, Vafaie J, Scheinfeld NS. Skin infections in the elderly. Dermatol Clin. 2004;22(1):51-61.
10. Walton SF, Beroukas D, Roberts-Thomson P, Currie BJ. New insights into disease pathogenesis in crusted (Norwegian) scabies: the skin immune response in crusted scabies. Br J Dermatol. 2008;158(6):1247-1255.
11. Currie BJ, Huffam SE, O’Brien D, Walton S. Ivermectin for scabies. Lancet. 1997;
350(9090):1551.
12. Roberts LJ, Huffam SE, Walton SF, Currie BJ. Crusted scabies: clinical and immunological findings in seventy-eight patients and a review of the literature. J Infect. 2005;50(5):375-381.
13. Walton SF, Pizzutto S, Slender A, et al. Increased allergic immune response to Sarcoptes scabiei antigens in crusted versus ordinary scabies. Clin Vaccine Immunol. 2010;17(9):1428-1438.
14. Hengge UR, Jäger G, Currie BJ, Lupi O, Schwartz RA. Scabies: a ubiquitous neglected skin disease. Lancet Infect Dis. 2006;6(12):769-779.
15. Lin S, Farber J, Lado L. A case report of crusted scabies with methicillin-resistant Staphylococcus aureus bacteremia. J Am Geriatr Soc. 2009;57(9):1713-1714.
16. Bonomo RA, Jacobs M, Jacobs G, Graham R, Salata RA. Norwegian scabies and a toxic shock syndrome toxin 1-producing strain of Staphylococcus aureus endocarditis in a patient with trisomy 21. Clin Infect Dis. 1998;27(3):645-646.
17. Mansy H, Somorin A, el-Sherif M, Eze C, al-Dusari S, Filobbos P. Norwegian scabies complicated by fatal brain abscess in a renal transplant patient. Nephron. 1996;72(2):323-324.
18. Kartono F, Lee EW, Lanum D, Pham L, Maibach HI. Crusted Norwegian scabies in an adult with Langerhans cell histiocytosis: mishaps leading to systemic chemotherapy. Arch Dermatol. 2007;143(5):626-628.
19. Currie BJ, McCarthy JS. Permethrin and ivermectin for scabies. N Engl J Med. 2010;362(8):717-725.
20. Makigami K, Ohtaki N, Ishii N, Yasumura S. Risk factors of scabies in psychiatric and long-term care hospitals: a nationwide mail-in survey in Japan. J Dermatol. 2009;36(9):491-498.
21. McCroskey A, Rosh A. Scabies in emergency medicine. Medscape Reference Website. http://emedicine.medscape.com/article/785873-overview. Updated October 6, 2010. Accessed August 2, 2011.
22. Flinders DC, De Schweinitz P. Pediculosis and scabies. Am Fam Physician. 2004;69(2):341-348.
23. Falk ES, Eide TJ. Histologic and clinical findings in human scabies. Int J Dermatol. 1981;20(9):600-605.
24. Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines 2002. Centers for Disease Control and Prevention. MMWR Recomm Rep. 2002;51(RR-6):1-78.
25. Scheinfeld N. Controlling scabies in institutional settings: a review of medications, treatment models, and implementation. Am J Clin Dermatol. 2004;5(1):31-37.
26. Utsumi M, Makimoto K, Quroshi N, Ashida N. Types of infectious outbreaks and their impact in elderly care facilities: a review of the literature. Age Ageing. 2010;39(3):299-305.
27. Management of scabies outbreaks in California health care facilities. California Department of Public Health Division of Communicable Disease Control In Consultation with Licensing and Certification. March 2008. http://www.cdph.ca.gov/pubsforms/guidelines/
documents/mgmntofscabiesoutbreaks.pdf. Accessed July 29, 2011.


