Thyroid Disorders in Adolescence: Autoimmune Versus Biochemical Hyperthyroidism
 Graves Disease
Graves Disease
For the past month, a 14-year-old girl had increased appetite, heat intolerance, nervousness, insomnia, frequent and loose stools, palpitations, and fatigue. She had a 4-lb weight loss during that time. She denied urinary frequency. Her maternal grandmother had a goiter; the nature of which was unknown.
The patient’s skin was warm and moist, and her face flushed. She had mild exophthalmos, lid lag, and hand tremor. Heart rate was 110 beats per minute; respiration rate, 28 breaths per minute; and blood pressure, 120/70 mm Hg. The thyroid gland was diffusely enlarged but nontender. A bruit was heard over the enlarged gland.
Graves disease is caused by immunologic stimulation of the thyroid-stimulating hormone (TSH) receptor on the thyroid cell membrane, which results in excessive synthesis and secretion of thyroid hormone.1,2 The condition occurs in 0.02% of children and is the most common cause of thyrotoxicosis in children and adolescents.2,3 The peak incidence is between 10 and 15 years of age; it more commonly affects girls than boys.2,4 Graves disease is more common among persons of Asian descent, and affected children are usually tall; their bone age is advanced beyond their chronologic age, but their sexual maturation is normal.
Classic symptoms of Graves disease include weight loss in spite of a good appetite, irritability, emotional lability, nervousness, heat intolerance, palpitations, increased frequency of bowel movements, insomnia, and, in postpubertal girls, oligomenorrhea.1 Characteristic signs include diffuse enlargement of the thyroid gland, tachycardia, wide pulse pressure, excessive perspiration, hand tremor, exophthalmos, lid lag, lid retraction, and a rapid tendon reflex relaxation phase.1 A palpable thrill may be present. A bruit may be heard over the enlarged gland.
Laboratory studies typically show a markedly suppressed or undetectable serum TSH level and elevated free triiodothyronine (T3) and thyroxine (T4) levels. This patient had a TSH level lower than 0.01 mIU/L (normal, 0.7 to 6.4 mIU/L), a T3 level of 1 ng/dL (normal, 0.27 to 0.57 ng/dL), and a T4 level of 3 ng/dL (normal, 0.8 to 2.2 ng/dL). TSH receptor antibodies (antithyroglobulin and antithyroid peroxidase antibodies) are detected in most patients with Graves disease and were detected in this patient.2
Antithyroid medication (eg, propylthiouracil, methimazole) is the treatment of choice.2 For patents who relapse or do not respond to treatment because of poor adherence or intolerance, clinicians should consider alternate therapies, such as radioactive iodine and subtotal thyroidectomy.2 Potential complications of Graves disease include thyroid storm, cardiac arrhythmia, cardiac failure, periodic paralysis, orbitopathy, rhabdomyolysis, pseudotumor cerebri, thrombotic thrombocytopenic purpura, and cholestasis.
REFERENCES:
1. Leung AK, Pacaud D. A swelling in the neck. Am Fam Physician. 2006;73(10):1793-1794.
2. Kaguelidou F, Carel JC, Léger J. Graves’ disease in childhood: advances in management with antithyroid drug therapy. Horm Res. 2009;71(6):310-317.
3. Rivkees SA. Pediatric Graves’ disease: controversies in management. Horm Res Paediatr. 2010;74(5):305-311.
4. Bauer AJ. Approach to the pediatric patient with Graves’ disease: when is definitive therapy warranted? J Clin Endocrinol Metab. 2011;96(3):580-588.
Toxic Thyroid Adenoma
This 17-year-old girl had heat intolerance, irritability, insomnia, tremor, and increased frequency of bowel movements for the past 10 months. During that time, she had a good appetite but lost 5 lb. There was no family history of thyroid disease.
A smooth, firm, symmetrical nodule of 4 × 5 cm was noted on the right side of the thyroid gland; no bruit was heard over the goiter. The patient also had moist palms and hand tremor. She had no exophthalmos or lid lag. Her heart rate was 125 beats per minute and blood pressure was 120/80 mm Hg.
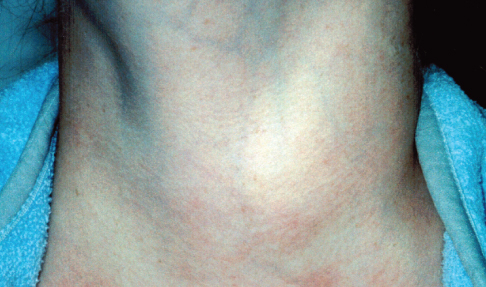
Laboratory results revealed a serum free thyroxine level of 4.5 ng/dL (normal, 0.8 to 2.2 ng/dL) and a thyroid-stimulating hormone (TSH) level lower than 0.01 mIU/L (normal, 0.7 to 6.4 mIU/L). Test results for TSH receptor antibodies (antithyroglobulin and antithyroid peroxidase antibodies) were negative.
Ultrasonography showed a nodule of isoechogenicity in right lobe of the thyroid. A radioactive iodine 123 thyroid scan showed a hot nodule in the right side of the thyroid, with suppressed uptake in the entire thyroid area. Fine-needle aspiration biopsy of the nodule confirmed the diagnosis of a toxic adenoma of the thyroid gland (Plummer disease).
Single thyroid nodules are uncommon in childhood and adolescence.1 Thyroid nodules may be nonfunctioning—as is the case for most benign adenomas—or functioning (toxic thyroid adenoma). In functioning thyroid nodules, thyrocytes produce thyroid hormones independently of serum TSH and in the absence of TSH receptor antibodies, as illustrated in this case.2 The autonomous secretion of thyroid hormones leads to thyrotoxicosis and TSH suppression.2
Toxic thyroid adenoma is a benign neoplasm that is associated with a somatic gain-of-function mutation in the thyrotropin receptor gene (TSHR) or the adenylate cyclase-stimulating G a protein gene (GNAS).3-5 The condition is more common in iodine-deficient regions.4
Patients with toxic thyroid adenoma have biochemical evidence of hyperthyroidism. However, they have milder and fewer toxic symptoms than patients with Graves disease.1 Lack of ocular changes (eg, exophthalmos and lid lag) is an important clinical feature of toxic thyroid adenoma that differentiates it from Graves disease. Like Graves disease, toxic thyroid adenoma can be complicated by periodic paralysis.2
REFERENCES:
1. Leung AK, McArthur RG, Dawson J, Seagram G. Unilateral thyroid enlargement with hyperthyroidism. Am J Dis Child. 1980;134(9):890-891.
2. Tagami T, Usui T, Shimatsu A, Naruse M. Toxic thyroid adenoma presenting as hypokalemic periodic paralysis. Endocr J. 2007;54(5):797-803.
3. Kohn B, Grasberger H, Lam LL, et al. A somatic gain-of-function mutation in the thyrotropin receptor gene producing a toxic adenoma in an infant. Thyroid. 2009;19(2):187-191.
4. Nanba K, Usui T, Minamiguchi S, et al. Two rare TSH receptor amino acid substitutions in toxic thyroid adenomas. Endocr J. 2012;59(1):13-19.
5. Zakavi SR, Mousavi Z, Davachi B. Comparison of four different protocols of I-131 therapy for treating single toxic thyroid nodule. Nucl Med Commun. 2009;30(2):169-175.


